Dose-exposure-IGF-I response of once-weekly somapacitan in adults with GH deficiency
- PMID: 35521713
- PMCID: PMC9175552
- DOI: 10.1530/EJE-21-1167
Dose-exposure-IGF-I response of once-weekly somapacitan in adults with GH deficiency
Abstract
Objective: Growth hormone (GH) replacement therapy in patients with adult growth hormone deficiency (AGHD) is individually titrated due to variable dose-responses among patients. The aim of this study was to provide clinical guidance on dosing and titration of the novel long-acting GH derivative somapacitan based on analyses of somapacitan dose-insulin-like growth factor I (IGF-I) responses in AGHD patients.
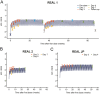
Design: Analyses of dosing information, 4364 somapacitan concentration samples and 4880 IGF-I samples from 330 AGHD patients treated with somapacitan in three phase 3 trials.
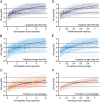
Methods: Pharmacokinetic/pharmacodynamic modelling was used to evaluate starting dose groups by age and oral oestrogen therapy, characterise the dose-IGF-I response in the overall AGHD population and patient subgroups, predict the IGF-I response to dose changes and simulate missed dosing.
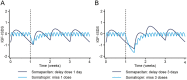
Results: The analyses supported the clinical recommendations of higher starting doses for younger patients and women on oral oestrogen replacement therapy. For patients switching from daily GH treatment, the mean maintenance dose ratio between somapacitan (mg/week) and somatropin (mg/day) was predicted to be 8.2 (observed interquartile range of 6.7-9.1). Simulations of IGF-I SDS profiles confirmed the appropriate time for IGF-I sampling to be 3-4 days after somapacitan dosing and supported somapacitan administration with up to 3 days delay in case of missed dosing. Subgroup analyses characterised the dose-exposure-IGF-I response in patient subgroups and indicated that dose requirements are mainly influenced by sex and oral oestrogen treatment.
Conclusions: This study extends the knowledge of the somapacitan dose-IGF-I response and provides information on clinical dosing of once-weekly somapacitan in patients with AGHD.
Figures