Emicizumab state-of-the-art update
- PMID: 35521723
- PMCID: PMC9321850
- DOI: 10.1111/hae.14524
Emicizumab state-of-the-art update
Abstract
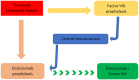
Introduction: Emicizumab is a bispecific monoclonal antibody developed to address the unmet needs of clotting factor replacement therapy and has become the benchmark for optimal prophylaxis in managing patients with haemophilia A with inhibitors. We describe the emicizumab rollout and pharmacokinetic strategies and their use in paediatric patients.
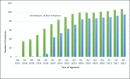
Methods: The evolving real-world experience in using emicizumab has confirmed its safety, efficacy and pharmacokinetic profile in paediatric, adolescent and adult patients receiving emicizumab at various prophylactic dosing regimens. The emicizumab current global rollout includes over 100 countries with 29 low to middle-income countries accessing emicizumab through the World Federation of Haemophilia (WFH) Humanitarian Aid Program. The diversity of emicizumab dosing and pharmacokinetic tools such as the Calibra® and the WAPPS-Hemo platforms make it possible to achieve prophylaxis goals in line with the WFH Haemophilia treatment guidelines recommendations, with minimal drug wastage. The emerging experience from long term clinical trials and long-term real-world follow-up confirm the safety, efficacy, and pharmacokinetic profile of emicizumab in paediatric haemophilia A patients. A few questions, including inhibitor recurrence, concurrent use of emicizumab with various replacement therapies and inhibitor eradication, are being addressed through multiple ongoing clinical studies.
Conclusion: The current global rollout of emicizumab is remarkable, and versatile dosing regimens and evolving pharmacokinetic tools such as the Calibra® and WAPPS-Hemo platforms make it a treatment choice available also for pharmacokinetic guided personalised treatment. Data from paediatric studies are consistent with those seen in adolescent and adult Haemophilia A.
Keywords: emicizumab; haemophilia A; paediatric; pharmacokinetic; real-world evidence; rollout.
© 2022 The Authors. Haemophilia published by John Wiley & Sons Ltd.
Figures
References
-
- Mahlangu J. An update of the current pharmacotherapeutic armamentarium for hemophilia A. Expert Opin Pharmacother. 2022;23:129‐138. - PubMed
-
- Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab Prophylaxis in Hemophilia A with Inhibitors. N Engl J Med. 2017;377:809‐818. - PubMed
-
- Mahlangu J, Oldenburg J, Paz‐Priel I, et al. Emicizumab Prophylaxis in Patients Who Have Hemophilia A without Inhibitors. N Engl J Med. 2018;379:811‐822. - PubMed
-
- Pipe SW, Shima M, Lehle M, et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open‐label, non‐randomised phase 3 study. Lancet Haematol. 2019;6:e295‐e305. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

