Azetidine-2-Carboxylic Acid-Induced Oligodendrogliopathy: Relevance to the Pathogenesis of Multiple Sclerosis
- PMID: 35521963
- PMCID: PMC9123080
- DOI: 10.1093/jnen/nlac028
Azetidine-2-Carboxylic Acid-Induced Oligodendrogliopathy: Relevance to the Pathogenesis of Multiple Sclerosis
Abstract
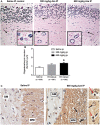
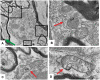
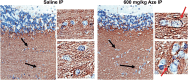
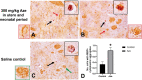
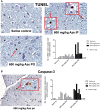
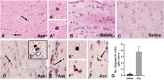
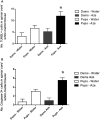
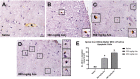
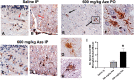
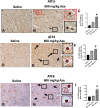
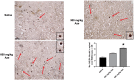
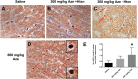
The naturally occurring imino acid azetidine-2-carboxylic acid (Aze) is consumed by humans and can be misincorporated in place of proline in myelin basic protein (MBP) in vitro. To determine Aze effects on the mammalian CNS in vivo, adult CD1 mice were given Aze orally or intraperitoneally. Clinical signs reminiscent of MBP-mutant mice occurred with 600 mg/kg Aze exposure. Aze induced oligodendrocyte (OL) nucleomegaly and nucleoplasm clearing, dilated endoplasmic reticulum, cytoplasmic vacuolation, abnormal mitochondria, and Aze dose-dependent apoptosis. Immunohistochemistry demonstrated myelin blistering and nuclear translocation of unfolded protein response (UPR)/proinflammatory molecules (ATF3, ATF4, ATF6, eIF2α, GADD153, NFκB, PERK, XBP1), MHC I expression, and MBP cytoplasmic aggregation in OL. There were scattered microglial nodules in CNS white matter (WM); other CNS cells appeared unaffected. Mice given Aze in utero and postnatally showed more marked effects than their dams. These OL, myelin, and microglial alterations are found in normal-appearing WM (NAWM) in multiple sclerosis (MS) patients. Thus, Aze induces a distinct oligodendrogliopathy in mice that recapitulates MS NAWM pathology without leukocyte infiltration. Because myelin proteins are relatively stable throughout life, we hypothesize that Aze misincorporation in myelin proteins during myelinogenesis in humans results in a progressive UPR that may be a primary process in MS pathogenesis.
Keywords: Autoimmunity; Azetidine-2-carboxylic acid; Multiple sclerosis; Myelin basic protein; Oligodendrocyte; Proline; Unfolded protein response.
© 2022 American Association of Neuropathologists, Inc. All rights reserved.
Figures



References
-
- Rubenstein E. Misincorporation of the proline analog azetidine-2-carboxylic acid in the pathogenesis of multiple sclerosis: A hypothesis. J Neuropathol Exp Neurol 2008;67:1035–40 - PubMed
-
- Sobel RA. A novel unifying hypothesis of multiple sclerosis. J Neuropathol Exp Neurol 2008;67:1032–4 - PubMed
-
- Rubenstein E, Zhou H, Krasinska KM, et al.Azetidine-2-carboxylic acid in garden beets (Beta vulgaris). Phytochemistry 2006;67:898–903 - PubMed
-
- Central Intelligence Agency. The World Factbook, Field Listing, Agriculture Products. Washington, DC: Central Intelligence Agency; 2021.
-
- Simpson S Jr, Wang W, Otahal P, et al.Latitude continues to be significantly associated with the prevalence of multiple sclerosis: An updated meta-analysis. J Neurol Neurosurg Psychiatry 2019;90:1193–200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

