Super-Resolution Vibrational Imaging Using Expansion Stimulated Raman Scattering Microscopy
- PMID: 35521971
- PMCID: PMC9284179
- DOI: 10.1002/advs.202200315
Super-Resolution Vibrational Imaging Using Expansion Stimulated Raman Scattering Microscopy
Abstract
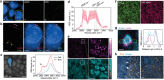
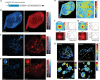
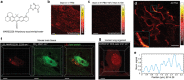
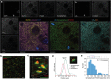
Stimulated Raman scattering (SRS) microscopy is an emerging technology that provides high chemical specificity for endogenous biomolecules and can circumvent common constraints of fluorescence microscopy including limited capabilities to probe small biomolecules and difficulty resolving many colors simultaneously. However, the resolution of SRS microscopy remains governed by the diffraction limit. To overcome this, a new technique called molecule anchorable gel-enabled nanoscale Imaging of Fluorescence and stimulated Raman scattering microscopy (MAGNIFIERS) that integrates SRS microscopy with expansion microscopy (ExM) is described. MAGNIFIERS offers chemical-specific nanoscale imaging with sub-50 nm resolution and has scalable multiplexity when combined with multiplex Raman probes and fluorescent labels. MAGNIFIERS is used to visualize nanoscale features in a label-free manner with CH vibration of proteins, lipids, and DNA in a broad range of biological specimens, from mouse brain, liver, and kidney to human lung organoid. In addition, MAGNIFIERS is applied to track nanoscale features of protein synthesis in protein aggregates using metabolic labeling of small metabolites. Finally, MAGNIFIERS is used to demonstrate 8-color nanoscale imaging in an expanded mouse brain section. Overall, MAGNIFIERS is a valuable platform for super-resolution label-free chemical imaging, high-resolution metabolic imaging, and highly multiplexed nanoscale imaging, thus bringing SRS to nanoscopy.
Keywords: Raman nanoscopy; chemical imaging; expansion microscopy; highly multiplexed nanoscale imaging; metabolic imaging.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare the following competing financial interest(s): Y.Z., A.K., and F.F. are inventors on several inventions related to ExM methods.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
