Going viral in the islet: mediators of SARS-CoV-2 entry beyond ACE2
- PMID: 35521990
- PMCID: PMC10622140
- DOI: 10.1530/JME-21-0282
Going viral in the islet: mediators of SARS-CoV-2 entry beyond ACE2
Abstract
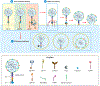
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Following initial infection of airway epithelia, SARS-CoV-2 invades a wide range of cells in multiple organs, including pancreatic islet cells. Diabetes is now recognised as a risk factor for severe COVID-19 outcomes, including hospitalisation and death. Additionally, COVID-19 is associated with a higher risk of new-onset diabetes and metabolic complications of diabetes. One mechanism by which these deleterious outcomes may occur is via the destruction of insulin-producing islet β cells, either directly by SARS-CoV-2 entry into β cells or indirectly due to inflammation and fibrosis in the surrounding microenvironment. While the canonical pathway of viral entry via angiotensin-converting enzyme 2 (ACE2) has been established as a major route of SARS-CoV-2 infection in the lung, it may not be solely responsible for viral entry into the endocrine pancreas. This is likely due to the divergent expression of viral entry factors among different tissues. For example, expression of ACE2 has not been unequivocally demonstrated in β cells. Thus, it is important to understand how other proteins known to be highly expressed in pancreatic endocrine cells may be involved in SARS-CoV-2 entry, with the view that these could be targeted to prevent the demise of the β cell in COVID-19. To that end, this review discusses alternate receptors of SARS-CoV-2 (CD147 and GRP78), as well as mediators (furin, TMPRSS2, cathepsin L, ADAM17, neuropilin-1, and heparan sulphate) that may facilitate SARS-CoV-2 entry into pancreatic islets independent of or in conjunction with ACE2.
Keywords: ACE2; ADAM17; CD147; COVID-19; GRP78; NRP1; SARS-CoV-2; TMPRSS2; cathepsin L; diabetes; furin; heparan sulfate; islet.
Conflict of interest statement
Declaration of Interest
The authors have no conflicts of interest to declare.
Figures
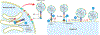

References
-
- Allagnat F, Fukaya M, Nogueira TC, Delaroche D, Welsh N, Marselli L, Marchetti P, Haefliger JA, Eizirik DL & Cardozo AK 2012. C/EBP homologous protein contributes to cytokine-induced pro-inflammatory responses and apoptosis in β-cells. Cell Death and Differentiation 19 1836–1846. (doi:10.1038/cdd.2012.67) - DOI - PMC - PubMed
-
- Amati F, Vancheri C, Latini A, Colona VL, Grelli S, D’Apice MR, Balestrieri E, Passarelli C, Minutolo A, Loddo S et al. 2020. Expression profiles of the SARS-CoV-2 host invasion genes in nasopharyngeal and oropharyngeal swabs of COVID-19 patients. Heliyon 6 e05143. (doi:10.1016/j.heliyon.2020.e05143) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

