Mapping Catalyst-Solvent Interplay in Competing Carboamination/Cyclopropanation Reactions
- PMID: 35522013
- PMCID: PMC9401068
- DOI: 10.1002/chem.202200399
Mapping Catalyst-Solvent Interplay in Competing Carboamination/Cyclopropanation Reactions
Abstract
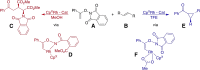
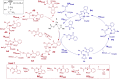
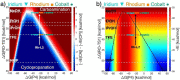
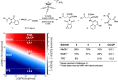
Group 9 metals, in particular RhIII complexes with cyclopentadienyl ligands, are competent C-H activation catalysts. Recently, a Cp*RhIII -catalyzed reaction of alkenes with N-enoxyphthalimides showed divergent outcome based on the solvent, with carboamination favored in methanol and cyclopropanation in 2,2,2-trifluoroethanol (TFE). Here, we create selectivity and activity maps capable of unravelling the catalyst-solvent interplay on the outcome of these competing reactions by analyzing 42 cyclopentadienyl metal catalysts, CpX MIII (M=Co, Rh, Ir). These maps not only can be used to rationalize previously reported experimental results, but also capably predict the behavior of untested catalyst/solvent combinations as well as aid in identifying experimental protocols that simultaneously optimize both catalytic activity and selectivity (solutions in the Pareto front). In this regard, we demonstrate how and why the experimentally employed Cp*RhIII catalyst represents an ideal choice to invoke a solvent-induced change in reactivity. Additionally, the maps reveal the degree to which even perceived minor changes in the solvent (e. g., replacing methanol with ethanol) influence the ratio of carboamination and cyclopropanation products. Overall, the selectivity and activity maps presented here provide a generalizable tool to create global pictures of anticipated reaction outcome that can be used to develop new experimental protocols spanning metal, ligand, and solvent space.
Keywords: C−H activation; density functional calculations; homogeneous catalysis; selectivity maps; volcano plots.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Electronic and Steric Tuning of a Prototypical Piano Stool Complex: Rh(III) Catalysis for C-H Functionalization.Acc Chem Res. 2018 Jan 16;51(1):170-180. doi: 10.1021/acs.accounts.7b00444. Epub 2017 Dec 22. Acc Chem Res. 2018. PMID: 29272106 Free PMC article. Review.
-
Solvent Mediating a Switch in the Mechanism for Rhodium(III)-Catalyzed Carboamination/Cyclopropanation Reactions between N-Enoxyphthalimides and Alkenes.Inorg Chem. 2017 May 1;56(9):5392-5401. doi: 10.1021/acs.inorgchem.7b00450. Epub 2017 Apr 17. Inorg Chem. 2017. PMID: 28414433
-
Chiral Cyclopentadienyls: Enabling Ligands for Asymmetric Rh(III)-Catalyzed C-H Functionalizations.Acc Chem Res. 2015 May 19;48(5):1308-18. doi: 10.1021/acs.accounts.5b00092. Epub 2015 Apr 17. Acc Chem Res. 2015. PMID: 25884306
-
Highly cis-selective Rh(I)-catalyzed cyclopropanation reactions.J Org Chem. 2011 Apr 15;76(8):2465-70. doi: 10.1021/jo102140z. Epub 2011 Mar 17. J Org Chem. 2011. PMID: 21413687
-
Diverse Approaches for Enantioselective C-H Functionalization Reactions Using Group 9 Cpx MIII Catalysts.Chemistry. 2020 Jun 10;26(33):7346-7357. doi: 10.1002/chem.201905417. Epub 2020 Apr 9. Chemistry. 2020. PMID: 31994236 Review.
Cited by
-
Enantioselective Cobalt(III)-Catalyzed [4 + 1] Annulation of Benzamides: Cyclopropenes as One-Carbon Synthons.J Am Chem Soc. 2025 May 7;147(18):15041-15049. doi: 10.1021/jacs.4c16953. Epub 2025 Apr 28. J Am Chem Soc. 2025. PMID: 40293123 Free PMC article.
-
Capturing Dichotomic Solvent Behavior in Solute-Solvent Reactions with Neural Network Potentials.J Chem Theory Comput. 2024 Dec 10;20(23):10350-10361. doi: 10.1021/acs.jctc.4c01201. Epub 2024 Nov 21. J Chem Theory Comput. 2024. PMID: 39570795 Free PMC article.
References
-
- Huang Z., Lim H. N., Mo F., Young M. C., Dong G., Chem. Soc. Rev. 2015, 44, 7764–7786. - PubMed
-
- Sperger T., Sanhueza I. A., Kalvet I., Schoenebeck F., Chem. Rev. 2015, 115, 9532–9586. - PubMed
-
- Tiwari V. K., Kapur M., Org. Biomol. Chem. 2019, 17, 1007–1026. - PubMed
-
- Newton C. G., Wang S.-G., Oliveira C. C., Cramer N., Chem. Rev. 2017, 117, 8908–8976. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

