Phosphatidylcholine:diacylglycerol cholinephosphotransferase's unique regulation of castor bean oil quality
- PMID: 35522031
- PMCID: PMC9342994
- DOI: 10.1093/plphys/kiac209
Phosphatidylcholine:diacylglycerol cholinephosphotransferase's unique regulation of castor bean oil quality
Abstract
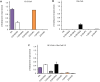
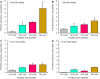
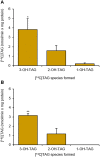
Castor bean (Ricinus communis) seed oil (triacylglycerol [TAG]) is composed of ∼90% of the industrially important ricinoleoyl (12-hydroxy-9-octadecenoyl) groups. Here, phosphatidylcholine (PC):diacylglycerol (DAG) cholinephosphotransferase (PDCT) from castor bean was biochemically characterized and compared with camelina (Camelina sativa) PDCT. DAGs with ricinoleoyl groups were poorly used by Camelina PDCT, and their presence inhibited the utilization of DAG with "common" acyl groups. In contrast, castor PDCT utilized DAG with ricinoleoyl groups similarly to DAG with common acyl groups and showed a 10-fold selectivity for DAG with one ricinoleoyl group over DAG with two ricinoleoyl groups. Castor DAG acyltransferase2 specificities and selectivities toward different DAG and acyl-CoA species were assessed and shown to not acylate DAG without ricinoleoyl groups in the presence of ricinoleoyl-containing DAG. Eighty-five percent of the DAG species in microsomal membranes prepared from developing castor endosperm lacked ricinoleoyl groups. Most of these species were predicted to be derived from PC, which had been formed by PDCT in exchange with DAG with one ricinoleoyl group. A scheme of the function of PDCT in castor endosperm is proposed where one ricinoleoyl group from de novo-synthesized DAG is selectivity transferred to PC. Nonricinoleate DAG is formed and ricinoleoyl groups entering PC are re-used either in de novo synthesis of DAG with two ricinoleoyl groups or in direct synthesis of triricinoleoyl TAG by PDAT. The PC-derived DAG is not used in TAG synthesis but is proposed to serve as a substrate in membrane lipid biosynthesis during oil deposition.
© The Author(s) 2022. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

