Visual memory failure presages conversion to MELAS phenotype
- PMID: 35522125
- PMCID: PMC9186137
- DOI: 10.1002/acn3.51564
Visual memory failure presages conversion to MELAS phenotype
Abstract
Objective: To examine the correlation between verbal and visual memory function and correlation with brain metabolites (lactate and N-Acetylaspartate, NAA) in individuals with mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS).
Methods: Memory performance and brain metabolites (ventricular lactate, occipital lactate, and occipital NAA) were examined in 18 MELAS, 58 m.3243A > G carriers, and 20 familial controls. Measures included the Selective Reminding Test (verbal memory), Benton Visuospatial Retention Test (visual memory), and MR Spectroscopy (NAA, Lactate). ANOVA, chi-squared/Fisher's exact tests, paired t-tests, Pearson correlations, and Spearman correlations were used.
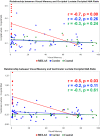
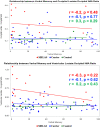
Results: When compared to carriers and controls, MELAS patients had the: (1) most impaired memory functions (Visual: p = 0.0003; Verbal: p = 0.02), (2) greatest visual than verbal memory impairment, (3) highest brain lactate levels (p < 0.0001), and (4) lowest brain NAA levels (p = 0.0003). Occipital and ventricular lactate to NAA ratios correlated significantly with visual memory performance (p ≤ 0.001). Higher lactate levels (p ≤ 0.01) and lower NAA levels (p = 0.0009) correlated specifically with greater visual memory dysfunction in MELAS. There was little or no correlation with verbal memory.
Interpretation: Individuals with MELAS are at increased risk for impaired memory. Although verbal and visual memory are both affected, visual memory is preferentially affected and more clearly associated with brain metabolite levels. Preferential involvement of posterior brain regions is a distinctive clinical signature of MELAS. We now report a distinctive cognitive phenotype that targets visual memory more prominently and earlier than verbal memory. We speculate that this finding in carriers presages a conversion to the MELAS phenotype.
© 2022 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Dr. Leaffer reports no financial and nonfinancial disclosure or conflict of interest. Dr. De Vivo reports no financial and nonfinancial disclosure or conflict of interest. Ms. Engelstad reports no financial and nonfinancial disclosure or conflict of interest. Dr. Fryer reports no financial and nonfinancial disclosure or conflict of interest. Dr. Gu reports no financial and nonfinancial disclosure or conflict of interest. Dr. Shungu reports no financial and nonfinancial disclosure or conflict of interest. Dr. Hirano reports no financial and nonfinancial disclosure of conflict of interests. Dr. DiMauro reports no financial and nonfinancial disclosure or conflict of interest. Dr. Hinton report no financial and nonfinancial disclosure or conflict of interest.
Figures
Similar articles
-
Metabolic abnormality in acute stroke-like lesion and its relationship with focal cerebral blood flow in patients with MELAS: Evidence from proton MR spectroscopy and arterial spin labeling.Mitochondrion. 2021 Jul;59:276-282. doi: 10.1016/j.mito.2021.06.012. Epub 2021 Jun 27. Mitochondrion. 2021. PMID: 34186261
-
Mitochondrial dysfunction and cerebral metabolic abnormalities in patients with mitochondrial encephalomyopathy subtypes: Evidence from proton MR spectroscopy and muscle biopsy.CNS Neurosci Ther. 2017 Aug;23(8):686-697. doi: 10.1111/cns.12714. Epub 2017 Jul 11. CNS Neurosci Ther. 2017. PMID: 28695670 Free PMC article.
-
Cerebral metabolic abnormalities in A3243G mitochondrial DNA mutation carriers.Neurology. 2014 Mar 4;82(9):798-805. doi: 10.1212/WNL.0000000000000169. Epub 2014 Jan 29. Neurology. 2014. PMID: 24477106 Free PMC article.
-
[Higher Brain Dysfunction in Mitochondrial Myopathy, Encephalopathy, Lactic Acidosis and Stroke-Like Episodes (MELAS)].Brain Nerve. 2016 Feb;68(2):151-7. doi: 10.11477/mf.1416200366. Brain Nerve. 2016. PMID: 26873235 Review. Japanese.
-
MELAS syndrome: Clinical manifestations, pathogenesis, and treatment options.Mol Genet Metab. 2015 Sep-Oct;116(1-2):4-12. doi: 10.1016/j.ymgme.2015.06.004. Epub 2015 Jun 15. Mol Genet Metab. 2015. PMID: 26095523 Review.
Cited by
-
Cognitive impairment profile in patients with the m.3243A> G variant in mitochondrial DNA.BMC Neurol. 2025 Jul 31;25(1):316. doi: 10.1186/s12883-025-04325-y. BMC Neurol. 2025. PMID: 40745599 Free PMC article.
References
-
- Pavlakis SG, Phillips PC, DiMauro S, De Vivo DC, Rowland LP. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol. 1984;16(4):481‐488. - PubMed
-
- Chinnery P, Johnson M, Wardell T, et al. The epidemiology of pathogenic mitochondrial DNA mutations. Ann Neurol. 2000;48(2):188‐193. - PubMed
-
- Darin N, Oldfors A, Moslemi AR, Holme E, Tulinius M. The incidence of mitochondrial encephalomyopathies in childhood: clinical features and morphological, biochemical, and DNA abnormalities. Ann Neurol. 2001;49(3):377‐383. - PubMed
-
- Uusimaa J, Moilanen JS, Vainionpää L, et al. Prevalence, segregation, and phenotype of the mitochondrial DNA 3243A> G mutation in children. Ann Neurol. 2007;62(3):278‐287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical