Altered glycosylation in pancreatic cancer and beyond
- PMID: 35522218
- PMCID: PMC9086500
- DOI: 10.1084/jem.20211505
Altered glycosylation in pancreatic cancer and beyond
Abstract
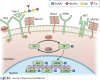
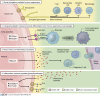
Pancreatic ductal adenocarcinoma (PDA) is one of the deadliest cancers and is projected to soon be the second leading cause of cancer death. Median survival of PDA patients is 6-10 mo, with the majority of diagnoses occurring at later, metastatic stages that are refractory to treatment and accompanied by worsening prognoses. Glycosylation is one of the most common types of post-translational modifications. The complex landscape of glycosylation produces an extensive repertoire of glycan moieties, glycoproteins, and glycolipids, thus adding a dynamic and tunable level of intra- and intercellular signaling regulation. Aberrant glycosylation is a feature of cancer progression and influences a broad range of signaling pathways to promote disease onset and progression. However, despite being so common, the functional consequences of altered glycosylation and their potential as therapeutic targets remain poorly understood and vastly understudied in the context of PDA. In this review, the functionality of glycans as they contribute to hallmarks of PDA are highlighted as active regulators of disease onset, tumor progression, metastatic capability, therapeutic resistance, and remodeling of the tumor immune microenvironment. A deeper understanding of the functional consequences of altered glycosylation will facilitate future hypothesis-driven studies and identify novel therapeutic strategies in PDA.
© 2022 Lumibao et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures

Similar articles
-
Role of tumor cell sialylation in pancreatic cancer progression.Adv Cancer Res. 2023;157:123-155. doi: 10.1016/bs.acr.2022.07.003. Epub 2022 Sep 27. Adv Cancer Res. 2023. PMID: 36725107 Free PMC article. Review.
-
COSMC knockdown mediated aberrant O-glycosylation promotes oncogenic properties in pancreatic cancer.Mol Cancer. 2015 May 29;14:109. doi: 10.1186/s12943-015-0386-1. Mol Cancer. 2015. PMID: 26021314 Free PMC article.
-
Glycoproteins and glycoproteomics in pancreatic cancer.World J Gastroenterol. 2016 Nov 14;22(42):9288-9299. doi: 10.3748/wjg.v22.i42.9288. World J Gastroenterol. 2016. PMID: 27895417 Free PMC article. Review.
-
The role of N-glycosylation modification in the pathogenesis of liver cancer.Cell Death Dis. 2023 Mar 29;14(3):222. doi: 10.1038/s41419-023-05733-z. Cell Death Dis. 2023. PMID: 36990999 Free PMC article. Review.
-
T-cell programming in pancreatic adenocarcinoma: a review.Cancer Gene Ther. 2017 Mar;24(3):106-113. doi: 10.1038/cgt.2016.66. Epub 2016 Dec 2. Cancer Gene Ther. 2017. PMID: 27910859 Review.
Cited by
-
GALNT12 suppresses the bone-specific prostate cancer metastasis by activating BMP pathway via the O-glycosylation of BMPR1A.Int J Biol Sci. 2024 Jan 27;20(4):1297-1313. doi: 10.7150/ijbs.91925. eCollection 2024. Int J Biol Sci. 2024. PMID: 38385080 Free PMC article.
-
A glycosylation-related signature predicts survival in pancreatic cancer.Aging (Albany NY). 2023 Nov 30;15(23):13710-13737. doi: 10.18632/aging.205258. Epub 2023 Nov 30. Aging (Albany NY). 2023. PMID: 38048216 Free PMC article.
-
Targeting glycans for CAR therapy: The advent of sweet CARs.Mol Ther. 2022 Sep 7;30(9):2881-2890. doi: 10.1016/j.ymthe.2022.07.006. Epub 2022 Jul 12. Mol Ther. 2022. PMID: 35821636 Free PMC article. Review.
-
Cyst fluid glycoproteins accurately distinguishing malignancies of pancreatic cystic neoplasm.Signal Transduct Target Ther. 2023 Oct 18;8(1):406. doi: 10.1038/s41392-023-01645-8. Signal Transduct Target Ther. 2023. PMID: 37848412 Free PMC article.
-
Altered glycosylation in cancer: molecular functions and therapeutic potential.Cancer Commun (Lond). 2024 Nov;44(11):1316-1336. doi: 10.1002/cac2.12610. Epub 2024 Sep 21. Cancer Commun (Lond). 2024. PMID: 39305520 Free PMC article. Review.
References
-
- Abdulkhalek, S., and Szewczuk M.R.. 2013. Neu1 sialidase and matrix metalloproteinase-9 cross-talk regulates nucleic acid-induced endosomal TOLL-like receptor-7 and -9 activation, cellular signaling and pro-inflammatory responses. Cell Signal. 25:2093–2105. 10.1016/j.cellsig.2013.06.010 - DOI - PubMed
-
- Almaraz, R.T., Tian Y., Bhattarcharya R., Tan E., Chen S.H., Dallas M.R., Chen L., Zhang Z., Zhang H., Konstantopoulos K., and Yarema K.J.. 2012. Metabolic flux increases glycoprotein sialylation: Implications for cell adhesion and cancer metastasis. Mol. Cell Proteomics. 11:M112.017558. 10.1074/mcp.M112.017558 - DOI - PMC - PubMed

