Adequacy of Serial Self-performed SARS-CoV-2 Rapid Antigen Detection Testing for Longitudinal Mass Screening in the Workplace
- PMID: 35522284
- PMCID: PMC9077488
- DOI: 10.1001/jamanetworkopen.2022.10559
Adequacy of Serial Self-performed SARS-CoV-2 Rapid Antigen Detection Testing for Longitudinal Mass Screening in the Workplace
Abstract
Importance: Longitudinal mass testing using rapid antigen detection tests (RADT) for serial screening of asymptomatic persons has been proposed for preventing SARS-CoV-2 community transmission. The feasibility of this strategy relies on accurate self-testing.
Objective: To quantify the adequacy of serial self-performed SARS-CoV-2 RADT testing in the workplace, in terms of the frequency of correct execution of procedural steps and accurate interpretation of the range of possible RADT results.
Design, setting, and participants: This prospective repeated cross-sectional study was performed from July to October 2021 at businesses with at least 2 active cases of SARS-CoV-2 infection in Montreal, Canada. Participants included untrained persons in their workplace, not meeting Public Health quarantine criteria (ie, required quarantine for 10 days after a moderate-risk contact with someone infected with SARS-CoV-2). Interpretation and performance were compared between participants who received instructions provided by the manufacturer vs those who received modified instructions that were informed by the most frequent or most critical errors we observed. Data were analyzed from October to November 2021.
Exposures: RADT testing using a modified quick reference guide compared with the original manufacturer's instructions.
Main outcomes and measures: The main outcome was the difference in correctly interpreted RADT results. Secondary outcomes included difference in proportions of correctly performed procedural steps. Additional analyses, assessed among participants with 2 self-testing visits, compared the second self-test visit with the first self-test visit using the same measures.
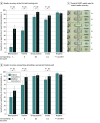
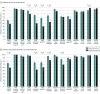
Results: Overall, 1892 tests were performed among 647 participants, of whom 278 participants (median [IQR] age, 43 [31-55] years; 156 [56.1%] men) had at least 1 self-testing visit. For self-test visit 1, significantly better accuracy in test interpretation was observed among participants using the modified quick reference guide than those using the manufacturer's instructions for reading results that were weak positive (64 of 115 participants [55.6%] vs 20 of 163 participants [12.3%]; difference, 43.3 [95% CI, 33.0-53.8] percentage points), positive (103 of 115 participants [89.6%] vs 84 of 163 participants [51.5%]; difference, 38.1 [95% CI, 28.5-47.5] percentage points), strong positive (219 of 229 participants [95.6%] vs 274 of 326 participants [84.0%]; difference, 11.6 [95% CI, 6.8-16.3] percentage points), and invalid (200 of 229 participants [87.3%] vs 252 of 326 participants [77.3%]; difference, 10.0 [95% CI, 3.8-16.3] percentage points). Use of the modified guide was associated with improvements on self-test visit 2 for results that were weak positive (difference, 15.4 [95% CI, 0.7-30.1] percentage points), positive (difference, 19.0 [95% CI, 7.2-30.9] percentage points), and invalid (difference, 8.0 [95% CI, 0.8-15.4] percentage points). For procedural steps identified as critical for test validity, adherence to procedural testing steps did not differ meaningfully according to instructions provided or reader experience.
Conclusions and relevance: In this cross-sectional study of self-performed SARS-CoV-2 RADT in an intended-use setting, a modified quick reference guide was associated with significantly improved accuracy in RADT interpretations.
Conflict of interest statement
Figures
Similar articles
-
Effectiveness and cost-effectiveness of four different strategies for SARS-CoV-2 surveillance in the general population (CoV-Surv Study): a structured summary of a study protocol for a cluster-randomised, two-factorial controlled trial.Trials. 2021 Jan 8;22(1):39. doi: 10.1186/s13063-020-04982-z. Trials. 2021. PMID: 33419461 Free PMC article.
-
Systematic on-site testing for SARS-CoV-2 infection among asymptomatic essential workers in Montréal, Canada: a prospective observational and cost-assessment study.CMAJ Open. 2022 May 10;10(2):E409-E419. doi: 10.9778/cmajo.20210290. Print 2022 Apr-Jun. CMAJ Open. 2022. PMID: 35537749 Free PMC article.
-
A single-center experience on long-term clinical performance of a rapid SARS-CoV-2 Antigen Detection Test, STANDARD Q COVID-19 Ag Test.Sci Rep. 2023 Nov 27;13(1):20777. doi: 10.1038/s41598-023-48194-2. Sci Rep. 2023. PMID: 38012319 Free PMC article.
-
Effectiveness of rapid antigen testing for screening of asymptomatic individuals to limit the transmission of SARS-CoV-2: A rapid review.Rev Med Virol. 2022 Sep;32(5):e2350. doi: 10.1002/rmv.2350. Epub 2022 Mar 29. Rev Med Virol. 2022. PMID: 35348276 Free PMC article. Review.
-
Performance of different rapid antigen testing strategies for SARS-CoV-2: A living rapid review.Eur J Clin Invest. 2023 Nov;53(11):e14058. doi: 10.1111/eci.14058. Epub 2023 Jul 9. Eur J Clin Invest. 2023. PMID: 37424144 Review.
Cited by
-
Characteristics associated with SARS-CoV-2 testing, infection and vaccine uptake among essential non-healthcare workers in Montréal, 2021.Can Commun Dis Rep. 2024 Jun 28;50(6):223-232. doi: 10.14745/ccdr.v50i06a05. eCollection 2024 Jun 28. Can Commun Dis Rep. 2024. PMID: 39021377 Free PMC article.
-
The Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19: Antigen Testing (January 2023).Clin Infect Dis. 2024 Jun 27;78(7):e350-e384. doi: 10.1093/cid/ciad032. Clin Infect Dis. 2024. PMID: 36702617 Free PMC article.
-
Stopping syphilis transmission in Arctic communities through rapid diagnostic testing: The STAR study protocol.PLoS One. 2022 Sep 12;17(9):e0273713. doi: 10.1371/journal.pone.0273713. eCollection 2022. PLoS One. 2022. PMID: 36094912 Free PMC article.
-
Daily, self-test rapid antigen test to assess SARS-CoV-2 viability in de-isolation of patients with COVID-19.Front Med (Lausanne). 2022 Oct 19;9:922431. doi: 10.3389/fmed.2022.922431. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36341265 Free PMC article.
-
Timing and Predictors of Loss of Infectivity Among Healthcare Workers With Mild Primary and Recurrent COVID-19: A Prospective Observational Cohort Study.Clin Infect Dis. 2024 Mar 20;78(3):613-624. doi: 10.1093/cid/ciad535. Clin Infect Dis. 2024. PMID: 37675577 Free PMC article.
References
-
- World Health Organization . WHO Coronavirus (COVID-19) Dashboard. Accessed November 23, 2021. https://covid19.who.int
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

