Implementation of EACS vaccination recommendations among people living with HIV
- PMID: 35522383
- PMCID: PMC9074432
- DOI: 10.1007/s15010-022-01827-6
Implementation of EACS vaccination recommendations among people living with HIV
Abstract
Objectives: With modern combination antiretroviral Treatment (cART) a normal life expectancy among people living with HIV (PLWH) has become reality if started early enough prior to the onset of more pronounced immunodeficiency. Therefore, prevention measures against other infectious diseases among this vulnerable group have gained increased attention. Indeed, the EACS guidelines recommend vaccinations against HAV, HBV, HPV, Influenza, Neisseria meningitidis, Streptococcus pneumoniae and VZV in HIV-infected adults.
Methods: All PLWH under cART attending our ID outpatient clinic between April to June 2018, were assessed during consultation for vaccination status regarding pneumococcus, Hepatitis A and B, influenza, varicella, meningococcus and HPV using a pre-defined questionnaire, vaccination certificates and medical records. In addition, the cohort database was screened for Hepatitis A and B serology and HIV surrogate markers.
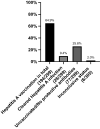
Results: A total of 305 PLWH (82.3% male, 17.7% female) was included, median age was 48 years (IQR 47-51). Median CD4 + T cell count was 543 (IQR 304-770), and for 297 (97.4%) PLWH CD4 + T cell count was ≥ 200/ul. The viral load was undetectable (< 40 copies/ml) in 289 (94.8%) cases. Highest vaccination rates were observed for HAV (87.4%), Streptococcus pneumoniae (77.4%) and Influenza (76.5%). 64.3% PLWH got vaccinated against HBV, whereas VZV vaccination only played a minor role, in the context of the high rate of cleared infections (99.0%). Lowest vaccination rates were detected for HPV (0%) and Neisseria meningitidis (3.0%).
Conclusions: Our data suggest that vaccination rates among PLWH are higher compared to the general German population. Implementation of EACS guidelines into daily routine though is not fully executed and the need for improving vaccination rates has to be emphasized. Centrally organized vaccination registers as well as electronic medical records could be helpful tools to detect a lack of vaccination coverage and send digital vaccination reminders particularly among risk groups.
Keywords: AIDS; HIV; Prevention; Vaccination.
© 2022. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures



References
-
- Hagemann C, et al. Specific varicella-related complications and their decrease in hospitalized children after the introduction of general varicella vaccination: results from a multicenter pediatric hospital surveillance study in Bavaria (Germany) Infect Dis Ther. 2019;8:597–611. doi: 10.1007/s40121-019-00273-6. - DOI - PMC - PubMed
-
- Robert-Koch-Institut, Online-Befragung von Klinikpersonal zur Influenza-Impfung (OKaPII-Studie), Epidemiologischen Bulletin 47/2016. 2016.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

