One-Pot, One-Step Synthesis of Drug-Loaded Magnetic Multimicelle Aggregates
- PMID: 35522527
- PMCID: PMC9121875
- DOI: 10.1021/acs.bioconjchem.2c00167
One-Pot, One-Step Synthesis of Drug-Loaded Magnetic Multimicelle Aggregates
Abstract
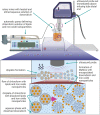
Lipid-based formulations provide a nanotechnology platform that is widely used in a variety of biomedical applications because it has several advantageous properties including biocompatibility, reduced toxicity, relative ease of surface modifications, and the possibility for efficient loading of drugs, biologics, and nanoparticles. A combination of lipid-based formulations with magnetic nanoparticles such as iron oxide was shown to be highly advantageous in a growing number of applications including magnet-mediated drug delivery and image-guided therapy. Currently, lipid-based formulations are prepared by multistep protocols. Simplification of the current multistep procedures can lead to a number of important technological advantages including significantly decreased processing time, higher reaction yield, better product reproducibility, and improved quality. Here, we introduce a one-pot, single-step synthesis of drug-loaded magnetic multimicelle aggregates (MaMAs), which is based on controlled flow infusion of an iron oxide nanoparticle/lipid mixture into an aqueous drug solution under ultrasonication. Furthermore, we prepared molecular-targeted MaMAs by directional antibody conjugation through an Fc moiety using Cu-free click chemistry. Fluorescence imaging and quantification confirmed that antibody-conjugated MaMAs showed high cell-specific targeting that was enhanced by magnetic delivery.
Conflict of interest statement
The authors declare the following competing financial interest(s): M.Z. is an employee of Imagion Biosystems. J.D.H. is a member of the Scientific Advisory Board, Imagion Biosystems.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

