Platelet transfusions in a murine model of neonatal polymicrobial sepsis: Divergent effects on inflammation and mortality
- PMID: 35522536
- PMCID: PMC11465244
- DOI: 10.1111/trf.16895
Platelet transfusions in a murine model of neonatal polymicrobial sepsis: Divergent effects on inflammation and mortality
Abstract
Background: Platelet transfusions (PTxs) are often given to septic preterm neonates at high platelet count thresholds in an attempt to reduce bleeding risk. However, the largest randomized controlled trial (RCT) of neonatal transfusion thresholds found higher mortality and/or major bleeding in infants transfused at higher thresholds. Using a murine model, we investigated the effects of adult PTx on neonatal sepsis-induced mortality, systemic inflammation, and platelet consumption.
Study design and methods: Polymicrobial sepsis was induced via intraperitoneal injection of cecal slurry preparations (CS1, 2, 3) into P10 pups. Two hours after infection, pups were transfused with washed adult Green Flourescent Protein (GFP+) platelets or control. Weights, platelet counts, and GFP% were measured before 4 and 24 h post-infection. At 24 h, blood was collected for quantification of plasma cytokines.
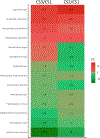
Results: The CS batches varied in 24 h mortality (11%, 73%, and 30% in CS1, 2, and 3, respectively), due to differences in bacterial composition. PTx had differential effects on sepsis-induced mortality and systemic inflammatory cytokines, increasing both in mice infected with CS1 (low mortality) and decreasing both in mice infected with CS2 and 3. In a mathematical model of platelet kinetics, the consumption of transfused adult platelets was higher than that of endogenous neonatal platelets, regardless of CS batch.
Discussion: Our findings support the hypothesis that transfused adult platelets are consumed faster than endogenous neonatal platelets in sepsis and demonstrate that PTx can enhance or attenuate neonatal inflammation and mortality in a model of murine polymicrobial sepsis, depending on the composition of the inoculum and/or the severity of sepsis.
Keywords: Cecal slurry; mathematical modeling; neonatal platelet transfusion; neonatal sepsis; neonatal thrombocytopenia; platelet consumption.
© 2022 AABB.
Conflict of interest statement
Figures



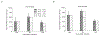
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

