Short communication: Feasibility of dengue vaccine to infect different human cell lines: An alternative potency test using HEK293T cells
- PMID: 35522661
- PMCID: PMC9075668
- DOI: 10.1371/journal.pone.0267653
Short communication: Feasibility of dengue vaccine to infect different human cell lines: An alternative potency test using HEK293T cells
Abstract
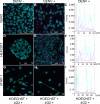
Dengue is caused by an arbovirus that belongs to the Flaviviridae family and there are four distinct, but close related, circulating serotypes. Dengue disease is of great importance for global public health, with vaccination being its main prophylactic measure. However, there is a paucity of biological models for evaluating tetravalent dengue vaccines. The aim of this study was to evaluate the susceptibility of human cell lines HEK293T and THP-1 to a commercial dengue vaccine and test the feasibility of this approach in the development of a potency assay with human cell lines, as a methodological alternative to the golden standard potency assay with VERO cells. In this context, we used a batch of the commercial vaccine Dengvaxia® (CYD-TDV) for the infection tests. We evaluated the presence of the vaccine virus in THP-1 cells, differentiated into macrophages (dTHP-1), and in HEK293T by confocal microscopy, using 4G2 pan-flavivirus antibody. Vaccine infectivity and potency were determined by immunocolorimetric assay using monoclonal antibodies specific for each serotype. The results indicated that the human strain HEK293T was responsive to the tetravalent vaccine, as shown by the presence of virus particles in the cell cytoplasm in a pattern similar to the one observed with VERO cells. Moreover, it was possible to determine the infectivity and potency values of each vaccine virus serotype in the HEK293T, with serotype 4 prevailing over the others. Thus, the human cell line HEK293T provides a potential candidate to be used in assays to determine potency and identity of tetravalent dengue vaccines.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Assessment of bivalent and tetravalent dengue vaccine formulations in flavivirus-naïve adults in Mexico.Hum Vaccin Immunother. 2014;10(10):2853-63. doi: 10.4161/21645515.2014.972131. Hum Vaccin Immunother. 2014. PMID: 25483647 Free PMC article. Clinical Trial.
-
Immunogenicity of the CYD tetravalent dengue vaccine using an accelerated schedule: randomised phase II study in US adults.BMC Infect Dis. 2018 Sep 21;18(1):475. doi: 10.1186/s12879-018-3389-x. BMC Infect Dis. 2018. PMID: 30241510 Free PMC article. Clinical Trial.
-
Immunogenicity and safety of simplified vaccination schedules for the CYD-TDV dengue vaccine in healthy individuals aged 9-50 years (CYD65): a randomised, controlled, phase 2, non-inferiority study.Lancet Infect Dis. 2021 Apr;21(4):517-528. doi: 10.1016/S1473-3099(20)30767-2. Epub 2020 Nov 16. Lancet Infect Dis. 2021. PMID: 33212067 Clinical Trial.
-
Development of a recombinant, chimeric tetravalent dengue vaccine candidate.Vaccine. 2015 Dec 10;33(50):7112-20. doi: 10.1016/j.vaccine.2015.11.022. Epub 2015 Nov 14. Vaccine. 2015. PMID: 26585500 Review.
-
From research to phase III: preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine.Vaccine. 2011 Sep 23;29(42):7229-41. doi: 10.1016/j.vaccine.2011.06.094. Epub 2011 Jul 13. Vaccine. 2011. PMID: 21745521 Review.
References
-
- WHO. Dengue. 2016. [cited 2021 April 09]. Available from: https://www.who.int/immunization/diseases/dengue/en/.
-
- WHO. Dengue and Severe dengue. 2021. [cited 2021 April 09]. Available from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
-
- Reiter P. Yellow fever and dengue: a threat to Europe? Euro Surveill. 2010; 15(10):pii:19509. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19509. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

