Frequency-dependent transition in power-law rheological behavior of living cells
- PMID: 35522746
- PMCID: PMC9075802
- DOI: 10.1126/sciadv.abn6093
Frequency-dependent transition in power-law rheological behavior of living cells
Abstract
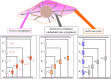
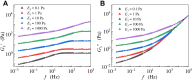
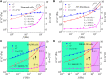
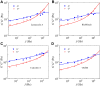
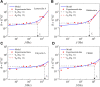
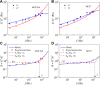
Living cells are active viscoelastic materials exhibiting diverse mechanical behaviors at different time scales. However, dynamical rheological characteristics of cells in frequency range spanning many orders of magnitude, especially in high frequencies, remain poorly understood. Here, we show that a self-similar hierarchical model can capture cell's power-law rheological characteristics in different frequency scales. In low-frequency scales, the storage and loss moduli exhibit a weak power-law dependence on frequency with same exponent. In high-frequency scales, the storage modulus becomes a constant, while the loss modulus shows a power-law dependence on frequency with an exponent of 1.0. The transition between low- and high-frequency scales is defined by a transition frequency based on cell's mechanical parameters. The cytoskeletal differences of different cell types or states can be characterized by changes in mechanical parameters in the model. This study provides valuable insights into potentially using mechanics-based markers for cell classification and cancer diagnosis.
Figures
Similar articles
-
Anisotropic power-law viscoelasticity of living cells is dominated by cytoskeletal network structure.Acta Biomater. 2024 May;180:197-205. doi: 10.1016/j.actbio.2024.04.002. Epub 2024 Apr 9. Acta Biomater. 2024. PMID: 38599439
-
A hierarchical cellular structural model to unravel the universal power-law rheological behavior of living cells.Nat Commun. 2021 Oct 18;12(1):6067. doi: 10.1038/s41467-021-26283-y. Nat Commun. 2021. PMID: 34663821 Free PMC article.
-
Soft glass rheology in liquid crystalline gels formed by a monodisperse dipeptide.J Phys Chem B. 2010 Jan 21;114(2):697-704. doi: 10.1021/jp9071394. J Phys Chem B. 2010. PMID: 20028007
-
Hierarchical network structure as the source of hierarchical dynamics (power-law frequency spectra) in living and non-living systems: How state-trait continua (body plans, personalities) emerge from first principles in biophysics.Neurosci Biobehav Rev. 2023 Nov;154:105402. doi: 10.1016/j.neubiorev.2023.105402. Epub 2023 Sep 22. Neurosci Biobehav Rev. 2023. PMID: 37741517 Review.
-
Fractional viscoelastic models for power-law materials.Soft Matter. 2020 Jul 8;16(26):6002-6020. doi: 10.1039/d0sm00354a. Soft Matter. 2020. PMID: 32638812 Review.
Cited by
-
Cancer cells impact the microrheology of endothelial cells during physical contact or through paracrine signalling.Sci Rep. 2025 Mar 8;15(1):8064. doi: 10.1038/s41598-025-92422-w. Sci Rep. 2025. PMID: 40055419 Free PMC article.
-
Acoustic Wave-Induced Stroboscopic Optical Mechanotyping of Adherent Cells.Adv Sci (Weinh). 2024 Apr;11(16):e2307929. doi: 10.1002/advs.202307929. Epub 2024 Feb 28. Adv Sci (Weinh). 2024. PMID: 38417124 Free PMC article.
-
Multi-Zone Visco-Node-Pore Sensing: A Microfluidic Platform for Multi-Frequency Viscoelastic Phenotyping of Single Cells.Adv Sci (Weinh). 2024 Nov;11(43):e2406013. doi: 10.1002/advs.202406013. Epub 2024 Sep 23. Adv Sci (Weinh). 2024. PMID: 39308179 Free PMC article.
-
Scaling-law mechanical marker for liver fibrosis diagnosis and drug screening through machine learning.Front Bioeng Biotechnol. 2024 Jul 16;12:1404508. doi: 10.3389/fbioe.2024.1404508. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39081332 Free PMC article.
-
Beyond stiffness: Multiscale viscoelastic features as biomechanical markers for assessing cell types and states.Biophys J. 2024 Jul 2;123(13):1869-1881. doi: 10.1016/j.bpj.2024.05.033. Epub 2024 Jun 4. Biophys J. 2024. PMID: 38835167 Free PMC article.
References
-
- Wu P.-H., Aroush D. R.-B., Asnacios A., Chen W.-C., Dokukin M. E., Doss B. L., Durand-Smet P., Ekpenyong A., Guck J., Guz N. V., Janmey P. A., Lee J. S. H., Moore N. M., Ott A., Poh Y.-C., Ros R., Sander M., Sokolov I., Staunton J. R., Wang N., Whyte G., Wirtz D., A comparison of methods to assess cell mechanical properties. Nat. Methods 15, 491–498 (2018). - PMC - PubMed
LinkOut - more resources
Full Text Sources

