Synthesis and In Vitro Studies of Photoactivatable Semisquaraine-type Pt(II) Complexes
- PMID: 35522899
- PMCID: PMC9131461
- DOI: 10.1021/acs.inorgchem.1c03957
Synthesis and In Vitro Studies of Photoactivatable Semisquaraine-type Pt(II) Complexes
Abstract
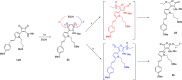
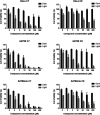
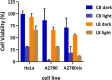
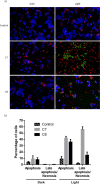
The synthesis, full characterization, photochemical properties, and cytotoxic activity toward cisplatin-resistant cancer cell lines of new semisquaraine-type Pt(II) complexes are presented. The synthesis of eight semisquaraine-type ligands has been carried out by means of an innovative, straightforward methodology. A thorough structural NMR and X-ray diffraction analysis of the new ligands and complexes has been done. Density functional theory calculations have allowed to assign the trans configuration of the platinum center. Through the structural modification of the ligands, it has been possible to synthesize some complexes, which have turned out to be photoactive at wavelengths that allow their activation in cell cultures and, importantly, two of them show remarkable solubility in biological media. Photodegradation processes have been studied in depth, including the structural identification of photoproducts, thus justifying the changes observed after irradiation. From biological assessment, complexes C7 and C8 have been demonstrated to behave as promising photoactivatable compounds in the assayed cancer cell lines. Upon photoactivation, both complexes are capable of inducing a higher cytotoxic effect on the tested cells compared with nonphotoactivated compounds. Among the observed results, it is remarkable to note that C7 showed a PI > 50 in HeLa cells, and C8 showed a PI > 40 in A2780 cells, being also effective over cisplatin-resistant A2780cis cells (PI = 7 and PI = 4, respectively). The mechanism of action of these complexes has been studied, revealing that these photoactivated platinum complexes would actually present a combined mode of action, a therapeutically potential advantage.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Chemistry of the Platinum Group Metals: Recent Developments; Hartley F. R., Ed.; Elsevier, 1991. ISBN-13: 978-0444881892. ISBN-10: 0444881891.
-
-
Some recent examples on some representative fields. Optics:
- Knedel T.-O.; Buss S.; Maisuls I.; Daniliuc C. G.; Schlüsener C.; Brandt P.; Weingart O.; Vollrath A.; Janiak C.; Strassert C. A. Encapsulation of Phosphorescent Pt(II) Complexes in Zn-Based Metal-Organic Frameworks toward Oxygen-Sensing Porous Materials. Inorg. Chem. 2020, 59, 7252–7264. 10.1021/acs.inorgchem.0c00678. - DOI - PubMed
- Fu G.; He Y.; Li W.; Wang B.; Lü X.; He H.; Wong W.-Y. Efficient polymer light-emitting diodes (PLEDs) based on chiral [Pt(ĈN)(N̂O)] complexes with near-infrared (NIR) luminescence and circularly polarized (CP) light. J. Mater. Chem. C 2019, 7, 13743–13747. 10.1039/C9TC04792A. - DOI
- Cabeza J. A.; Fernández-Colinas J. M.; García-Álvarez P.; González-Álvarez L.; Pérez-Carreño E. Reactivity of Amidinatosilylenes and Amidinatogermylenes with [PtMe2(η4-cod)]: cis- versus trans-[PtMe2L2] Complexes and Cyclometalation Reactions. Organometallics 2020, 39, 2026–2036. 10.1021/acs.organomet.0c00188. - DOI
- Kang S. K.; Hwang N.; Pak S.; Kim Y. K.; Yoon S. S. Platinum(II) Complexes Based on Phenylbenzoazole-Derived Ligands for Phosphorescent Organic Light-Emitting Diodes. J. Nanosci. Nanotechnol. 2020, 20, 589–593. 10.1166/jnn.2020.17244. - DOI - PubMed
- Peng K.; Einsele R.; Irmler P.; Winter R. F.; Schatzschneider U. The iClick Reaction of a BODIPY Platinum(II) Azido Complex with Electron-Poor Alkynes Provides Triazolate Complexes with Good 1O2 Sensitization Efficiency. Organometallics 2020, 39, 1423–1430. 10.1021/acs.organomet.0c00128. - DOI
- Zaitceva O.; Bénéteau V.; Ryabukhin D. S.; Eliseev I. I.; Kinzhalov M. A.; Louis B.; Vasilyev A. V.; Pale P. Cyclization of Aryl 3-Aryl Propynoates into 4-Arylcoumarins Catalyzed by Cyclometalated Platinum(II) Complexes. Tetrahedron 2020, 76, 131029–131037. 10.1016/j.tet.2020.131029. - DOI
- Ren J.; Cnudde M.; Brünink D.; Buss S.; Daniliuc C. G.; Liu L.; Fuchs H.; Strassert C. A.; Gao H. Y.; Doltsinis N. L. On-Surface Reactive Planarization of Pt(II) Complexes. Angew. Chem., Int. Ed. 2019, 58, 15396–15400. 10.1002/anie.201906247. - DOI - PMC - PubMed
- Chen Z.; Xue Y.; Gui M.; Wang C.; Wang F. Structural Isomerism Effect in Platinum(II) Acetylide-Based Supramolecular Polymers. Inorg. Chem. 2020, 59, 6481–6488. 10.1021/acs.inorgchem.0c00575. - DOI - PubMed
- Kobayashi A.; Kato M. Vapochromic Platinum(II) Complexes: Crystal Engineering toward Intelligent Sensing Devices. Eur. J. Inorg. Chem. 2014, 2014, 4469–4483. 10.1002/ejic.201402315. - DOI
- Díez Á.; Lalinde E.; Moreno M. T. Heteropolynuclear Cycloplatinated Complexes: Structural and Photophysical Properties. Coord. Chem. Rev. 2011, 255, 2426–2447. 10.1016/j.ccr.2010.12.024. - DOI
- Gao Z.; Han Y.; Gao Z.; Wang F. Multicomponent Assembled Systems Based on Platinum(II) Terpyridine Complexes. Acc. Chem. Res. 2018, 51, 2719–2729. 10.1021/acs.accounts.8b00340. - DOI - PubMed
- Fu T.-F.; Ao L.; Gao Z.-C.; Zhang X.-L.; Wang F. Advances on Supramolecular Assembly of Cyclometalated Platinum(II) Complexes. Chin. Chem. Lett. 2016, 27, 1147–1154. 10.1016/j.cclet.2016.06.054. - DOI
- Mauro M.; Aliprandi A.; Septiadi D.; Kehr N. S.; De Cola L. When Self-Assembly Meets Biology: Luminescent Platinum Complexes for Imaging Applications. Chem. Soc. Rev. 2014, 43, 4144–4166. 10.1039/C3CS60453E. - DOI - PubMed
-
-
-
Two recent examples:
- Nkabyo H. A.; Bosman G. W.; Luckay R. C.; Koch K. R. New E,Z platinum(II) complexes of asymmetrically di-substituted-acyl(aroyl)thioureas: Synthesis, characterization, photo-induced isomerism. Inorg. Chim. Acta 2020, 508, 119644–119651. 10.1016/j.ica.2020.119644. - DOI
- Yasuda J.; Inoue K.; Mizuno K.; Arai S.; Uehara K.; Kikuchi A.; Yan Y.-N.; Yamanishi K.; Kataoka Y.; Kato M.; Kawai A.; Kawamoto T. Photooxidation Reactions of Cyclometalated Palladium(II) and Platinum(II) Complexes. Inorg. Chem. 2019, 58, 15720–15725. 10.1021/acs.inorgchem.9b01492. - DOI - PubMed
-
-
- Johnstone T. C.; Suntharalingam K.; Lippard S. J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. 10.1021/acs.chemrev.5b00597. - DOI - PMC - PubMed
- Chen H. H.; Chen W.-C.; Liang Z.-D.; Tsai W.-B.; Long Y.; Aiba I.; Fu S.; Broaddus R.; Liu J.; Feun L. G.; Savaraj N.; Kuo M. T. Targeting Drug Transport Mechanisms for Improving Platinum-Based Cancer Chemotherapy. Expert Opin. Ther. Targets 2015, 19, 1307–1317. 10.1517/14728222.2015.1043269. - DOI - PMC - PubMed
- Ali I.; Wani W.; A. Wani K.; Haque A. Platinum Compounds: a Hope for Future Cancer Chemotherapy. Anti-Cancer Agents Med. Chem. 2013, 13, 296–306. 10.2174/1871520611313020016. - DOI - PubMed
-
-
Some recent examples:
- Shi H.; Imberti C.; Huang H.; Hands-Portman I.; Sadler P. J. Biotinylated photoactive Pt(iv) anticancer complexes. Chem. Commun. 2020, 56, 2320–2323. 10.1039/C9CC07845B. - DOI - PubMed
- Shi H.; Clarkson G. J.; Sadler P. J. Dual Action Photosensitive Platinum(II) Anticancer Prodrugs with Photoreleasable Azide Ligands. Inorg. Chim. Acta 2019, 489, 230–235. 10.1016/j.ica.2019.02.016. - DOI
- Presa A.; Vázquez G.; Barrios L. A.; Roubeau O.; Korrodi-Gregório L.; Pérez-Tomás R.; Gamez P. Photoactivation of the Cytotoxic Properties of Platinum(II) Complexes through Ligand Photoswitching. Inorg. Chem. 2018, 57, 4009–4022. 10.1021/acs.inorgchem.8b00146. - DOI - PubMed
- Mitra K. Platinum Complexes as Light Promoted Anticancer Agents: a Redefined Strategy for Controlled Activation. Dalton Trans. 2016, 45, 19157–19171. and references there in10.1039/C6DT03665A. - DOI - PubMed
- Shaili E. Platinum Anticancer Drugs and Photochemotherapeutic Agents: Recent Advances and Future Developments. Sci. Prog. 2014, 97, 20–40. and references there in10.3184/003685014X13904811808460. - DOI - PMC - PubMed
-
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

