Targeting DCLK1 overcomes 5-fluorouracil resistance in colorectal cancer through inhibiting CCAR1/β-catenin pathway-mediated cancer stemness
- PMID: 35522902
- PMCID: PMC9076011
- DOI: 10.1002/ctm2.743
Targeting DCLK1 overcomes 5-fluorouracil resistance in colorectal cancer through inhibiting CCAR1/β-catenin pathway-mediated cancer stemness
Abstract
Background: To date, 5-fluorouracil-based chemotherapy is very important for locally advanced or metastatic colorectal cancer (CRC). However, chemotherapy resistance results in tumor recurrence and metastasis, which is a major obstacle for treatment of CRC.
Methods: In the current research, we establish 5-fluorouracil resistant cell lines and explore the potential targets associated with 5-fluorouracil resistance in CRC. Moreover, we perform clinical specimen research, in vitro and in vivo experiments and molecular mechanism research, to reveal the biological effects and the mechanism of DCLK1 promoting 5-fluorouracil resistance, and to clarify the potential clinical value of DCLK1 as a target of 5-fluorouracil resistance in CRC.
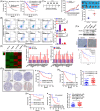
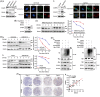
Results: We discover that doublecortin-like kinase 1 (DCLK1), a cancer stem cell maker, is correlated with 5-fluorouracil resistance, and functionally promotes cancer stemness and 5-fluorouracil resistance in CRC. Mechanistically, we elucidate that DCLK1 interacts with cell cycle and apoptosis regulator 1 (CCAR1) through the C-terminal domain, and phosphorylates CCAR1 at the Ser343 site, which is essential for CCAR1 stabilisation. Moreover, we find that DCLK1 positively regulates β-catenin signalling via CCAR1, which is responsible for maintaining cancer stemness. Subsequently, we prove that blocking β-catenin inhibits DCLK1-mediated 5-fluorouracil resistance in CRC cells. Importantly, we demonstrate that DCLK1 inhibitor could block CCAR1/β-catenin pathway-mediated cancer stemness and consequently suppresses 5-fluorouracil resistant CRC cells in vitro and in vivo.
Conclusions: Collectively, our findings reveal that DCLK1 promotes 5-fluorouracil resistance in CRC by CCAR1/β-catenin pathway-mediated cancer stemness, and suggest that targeting DCLK1 might be a promising method to eliminate cancer stem cells for overcoming 5-fluorouracil resistance in CRC.
Keywords: CCAR1; DCLK1; cancer stemness; chemoresistence; colorectal cancer; β-catenin.
© 2022 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures







Similar articles
-
DCLK1 mediated cooperative acceleration of EMT by avian leukosis virus subgroup J and Marek's disease virus via the Wnt/β-catenin pathway promotes tumor metastasis.J Virol. 2024 Nov 19;98(11):e0111224. doi: 10.1128/jvi.01112-24. Epub 2024 Oct 24. J Virol. 2024. PMID: 39445786 Free PMC article.
-
Doublecortin-like kinase 1 promotes stem cell-like properties through the Hippo-YAP pathway in prostate cancer.Int J Med Sci. 2025 Jan 1;22(2):460-472. doi: 10.7150/ijms.99062. eCollection 2025. Int J Med Sci. 2025. PMID: 39781521 Free PMC article.
-
ELF1's Role in Colorectal Cancer: Up-Regulating DCLK1 Expression to Propel Malignant Progression and Stemness Features.Discov Med. 2025 Feb;37(193):315-325. doi: 10.24976/Discov.Med.202537193.25. Discov Med. 2025. PMID: 39973554
-
The Essential Role of DCLK1 in Pathogenesis, Diagnostic Procedures and Prognostic Stratification of Colorectal Cancer.Anticancer Res. 2019 Jun;39(6):2689-2697. doi: 10.21873/anticanres.13394. Anticancer Res. 2019. PMID: 31177103 Review.
-
Role of DCLK1 in oncogenic signaling (Review).Int J Oncol. 2022 Nov;61(5):137. doi: 10.3892/ijo.2022.5427. Epub 2022 Sep 23. Int J Oncol. 2022. PMID: 36148883 Review.
Cited by
-
NDR1/FBXO11 promotes phosphorylation-mediated ubiquitination of β-catenin to suppress metastasis in prostate cancer.Int J Biol Sci. 2024 Sep 16;20(12):4957-4977. doi: 10.7150/ijbs.98907. eCollection 2024. Int J Biol Sci. 2024. PMID: 39309441 Free PMC article.
-
Deciphering treatment resistance in metastatic colorectal cancer: roles of drug transports, EGFR mutations, and HGF/c-MET signaling.Front Pharmacol. 2024 Jan 10;14:1340401. doi: 10.3389/fphar.2023.1340401. eCollection 2023. Front Pharmacol. 2024. PMID: 38269272 Free PMC article. Review.
-
Phytochemicals in Drug Discovery-A Confluence of Tradition and Innovation.Int J Mol Sci. 2024 Aug 13;25(16):8792. doi: 10.3390/ijms25168792. Int J Mol Sci. 2024. PMID: 39201478 Free PMC article. Review.
-
UVRAG Promotes Tumor Progression through Regulating SP1 in Colorectal Cancer.Cancers (Basel). 2023 Apr 27;15(9):2502. doi: 10.3390/cancers15092502. Cancers (Basel). 2023. PMID: 37173968 Free PMC article.
-
Crosstalk between colorectal CSCs and immune cells in tumorigenesis, and strategies for targeting colorectal CSCs.Exp Hematol Oncol. 2024 Jan 22;13(1):6. doi: 10.1186/s40164-024-00474-x. Exp Hematol Oncol. 2024. PMID: 38254219 Free PMC article. Review.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609–1618. - PubMed
-
- Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–572. - PubMed
-
- Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair‐deficient/microsatellite instability‐high metastatic colorectal cancer. J Clin Oncol. 2018;36:773–779. - PubMed
-
- Overman MJ, Kopetz S, McDermott RS, et al. Nivolumab ± ipilimumab in treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC) with and without high microsatellite instability (MSI‐H): CheckMate‐142 interim results. J Clin Oncol. 2016;34:3501.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
