NAT10-mediated mRNA N4-acetylcytidine modification promotes bladder cancer progression
- PMID: 35522942
- PMCID: PMC9076013
- DOI: 10.1002/ctm2.738
NAT10-mediated mRNA N4-acetylcytidine modification promotes bladder cancer progression
Abstract
Background: Dysregulation of the epitranscriptome causes abnormal expression of oncogenes in the tumorigenic process. Previous studies have shown that NAT10 can regulate mRNA translation efficiency through RNA acetylation. However, the role of NAT10-mediated acetylation modification in bladder cancer remains elusive.
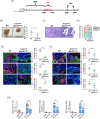
Methods: The clinical value of NAT10 was estimated according to NAT10 expression pattern based on TCGA data set and the tumor tissue array. Acetylated RNA immunoprecipitation sequencing was utilized to explore the role of NAT10 in mRNA ac4C modification. Translation efficiency and mRNA stability assay were applied to study the effect of NAT10-deletion on target genes. The nude mouse model and genetically engineered mice were conducted to further verify the characteristics of NAT10 in promoting BLCA progression and regulating downstream targets.
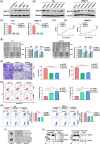
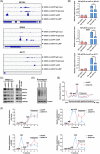
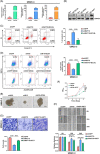
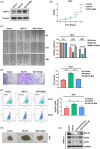
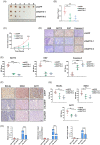
Results: NAT10 was essential for the proliferation, migration, invasion, survival and the stem-cell-like properties of bladder cancer cell lines. NAT10 was responsible for mRNA ac4C modification in BLCA cells, including BCL9L, SOX4 and AKT1. Deficient NAT10 in both xenograft and transgenic mouse models of bladder cancer reduced the tumor burden. Furthermore, acetylated RNA immunoprecipitation sequencing data and RNA immunoprecipitation qPCR results revealed that NAT10 is responsible for a set of ac4C mRNA modifications in bladder cancer cells. Inhibition of NAT10 led to a loss of ac4C peaks in these transcripts and represses the mRNA's stability and protein expression. Mechanistically, the ac4C reduction modification in specific regions of mRNAs resulting from NAT10 downregulation impaired the translation efficiency of BCL9L, SOX4 and AKT1 as well as the stability of BCL9L, SOX4.
Conclusions: In summary, these findings provide new insights into the dynamic characteristics of mRNA's post-transcriptional modification via NAT10-dependent acetylation and predict a role for NAT10 as a therapeutic target in bladder cancer.
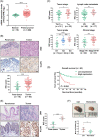
Highlights: NAT10 is highly expressed in BLCA patients and its abnormal level predicts bladder cancer progression and low overall survival rate. NAT10 is necessary and sufficient for BLCA tumourigenic properties. NAT10 is responsible for ac4C modification of target transcripts, including BCL9L, SOX4 and AKT1. NAT10 may serve as an effective and novel therapeutic target for BLCA.
Keywords: N4-acetylcytidine; NAT10; bladder cancer; mRNA.
© 2022 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures

Similar articles
-
Targeting N4-acetylcytidine suppresses hepatocellular carcinoma progression by repressing eEF2-mediated HMGB2 mRNA translation.Cancer Commun (Lond). 2024 Sep;44(9):1018-1041. doi: 10.1002/cac2.12595. Epub 2024 Jul 19. Cancer Commun (Lond). 2024. PMID: 39030964 Free PMC article.
-
Acetylation of Cytidine in mRNA Promotes Translation Efficiency.Cell. 2018 Dec 13;175(7):1872-1886.e24. doi: 10.1016/j.cell.2018.10.030. Epub 2018 Nov 15. Cell. 2018. PMID: 30449621 Free PMC article.
-
N-acetyltransferase 10 regulates alphavirus replication via N4-acetylcytidine (ac4C) modification of the lymphocyte antigen six family member E (LY6E) mRNA.J Virol. 2024 Jan 23;98(1):e0135023. doi: 10.1128/jvi.01350-23. Epub 2024 Jan 3. J Virol. 2024. PMID: 38169284 Free PMC article.
-
Emerging Role of NAT10 as ac4C Writer in Inflammatory Diseases: Mechanisms and Therapeutic Applications.Curr Drug Targets. 2025;26(4):282-294. doi: 10.2174/0113894501346709241202110834. Curr Drug Targets. 2025. PMID: 39633518 Review.
-
Emerging role of RNA acetylation modification ac4C in diseases: Current advances and future challenges.Biochem Pharmacol. 2023 Jul;213:115628. doi: 10.1016/j.bcp.2023.115628. Epub 2023 May 27. Biochem Pharmacol. 2023. PMID: 37247745 Review.
Cited by
-
Improvement of MASLD and MASH by suppression of hepatic N-acetyltransferase 10.Mol Metab. 2024 Nov;89:102030. doi: 10.1016/j.molmet.2024.102030. Epub 2024 Sep 16. Mol Metab. 2024. PMID: 39293565 Free PMC article.
-
N-acetyltransferase 10 promotes colon cancer progression by inhibiting ferroptosis through N4-acetylation and stabilization of ferroptosis suppressor protein 1 (FSP1) mRNA.Cancer Commun (Lond). 2022 Dec;42(12):1347-1366. doi: 10.1002/cac2.12363. Epub 2022 Oct 8. Cancer Commun (Lond). 2022. PMID: 36209353 Free PMC article.
-
N-acetyltransferase 10 affects the proliferation of intrahepatic cholangiocarcinoma and M2-type polarization of macrophages by regulating C-C motif chemokine ligand 2.J Transl Med. 2024 Sep 30;22(1):875. doi: 10.1186/s12967-024-05664-z. J Transl Med. 2024. PMID: 39350174 Free PMC article.
-
N4-acetylcytidine-dependent GLMP mRNA stabilization by NAT10 promotes head and neck squamous cell carcinoma metastasis and remodels tumor microenvironment through MAPK/ERK signaling pathway.Cell Death Dis. 2023 Nov 1;14(11):712. doi: 10.1038/s41419-023-06245-6. Cell Death Dis. 2023. PMID: 37914704 Free PMC article.
-
NAT10 Mediates XPO1 mRNA N4-acetylation and Promotes Drug Resistance of Myeloma Cells.J Cancer. 2024 Oct 14;15(19):6355-6363. doi: 10.7150/jca.101403. eCollection 2024. J Cancer. 2024. PMID: 39513105 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7‐34. - PubMed
-
- He YT, Li DJ, Liang D, et al. Incidence and mortality of bladder cancer in China, 2014. Chin J Oncol. 2018;40:647‐652. - PubMed
-
- Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder cancer: a review. JAMA. 2020;324:1980‐1991. - PubMed
-
- Chen Y, Hong T, Wang S, Mo J, Tian T, Zhou X. Epigenetic modification of nucleic acids: from basic studies to medical applications. Chem Soc Rev. 2017;46:2844‐2872. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
