Injectable Hydrogel Containing Cowpea Mosaic Virus Nanoparticles Prevents Colon Cancer Growth
- PMID: 35522951
- PMCID: PMC9840516
- DOI: 10.1021/acsbiomaterials.2c00284
Injectable Hydrogel Containing Cowpea Mosaic Virus Nanoparticles Prevents Colon Cancer Growth
Abstract
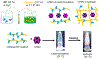
Despite advances in laparoscopic surgery combined with neoadjuvant and adjuvant therapy, colon cancer management remains challenging in oncology. Recurrence of cancerous tissue locally or in distant organs (metastasis) is the major problem in colon cancer management. Vaccines and immunotherapies hold promise in preventing cancer recurrence through stimulation of the immune system. We and others have shown that nanoparticles from plant viruses, such as cowpea mosaic virus (CPMV) nanoparticles, are potent immune adjuvants for cancer vaccines and serve as immunostimulatory agents in the treatment or prevention of tumors. While being noninfectious toward mammals, CPMV activates the innate immune system through recognition by pattern recognition receptors (PRRs). While the particulate structure of CPMV is essential for prominent immune activation, the proteinaceous architecture makes CPMV subject to degradation in vivo; thus, CPMV immunotherapy requires repeated injections for optimal outcome. Frequent intraperitoneal (IP) injections however are not optimal from a clinical point of view and can worsen the patient's quality of life due to the hospitalization required for IP administration. To overcome the need for repeated IP injections, we loaded CPMV nanoparticles in injectable chitosan/glycerophosphate (GP) hydrogel formulations, characterized their slow-release potential, and assessed the antitumor preventative efficacy of CPMV-in-hydrogel single dose versus soluble CPMV (single and prime-boost administration). Using fluorescently labeled CPMV-in-hydrogel formulations, in vivo release data indicated that single IP injection of the hydrogel formulation yielded a gel depot that supplied intact CPMV over the study period of 3 weeks, while soluble CPMV lasted only for one week. IP administration of the CPMV-in-hydrogel formulation boosted with soluble CPMV for combined immediate and sustained immune activation significantly inhibited colon cancer growth after CT26 IP challenge in BALB/c mice. The observed antitumor efficacy suggests that CPMV can be formulated in a chitosan/GP hydrogel to achieve prolonged immunostimulatory effects as single-dose immunotherapy against colon cancer recurrence. The present findings illustrate the potential of injectable hydrogel technology to accommodate plant virus nanoparticles to boost the translational development of effective antitumor immunotherapies.
Keywords: antitumor immunotherapy; chitosan; colon cancer recurrence; cowpea mosaic virus; in situ forming depot; plant virus; thermosensitive hydrogels.
Conflict of interest statement
The authors declare the following competing financial interest(s): Dr. N. F. Steinmetz is a co-founder of, has equity in, and has a financial interest with Mosaic ImmunoEngineering Inc.; Dr. N. F. Steinmetz serves as the Director, Board Member, Acting Chief Scientific Officer, and paid consultant to Mosaic. Dr. C. I. Nkanga declares no potential conflicts of interest.
Figures


References
-
- Cancer - WHO, World Health Organization 2022. https://www.who.int/News-Room/Fact-Sheets/Detail/Cancer (accessed Jan 29, 2022).
-
- Siegel RL; Miller KD; Fuchs HE; Jemal A Cancer Statistics, 2021. Ca-Cancer J. Clin 2021, 71, 7–33. - PubMed
-
- Torre LA; Bray F; Siegel RL; Ferlay J; Lortet-Tieulent J; Jemal A Global Cancer Statistics, 2012. Ca-Cancer J. Clin 2015, 65, 87–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
