Reversible Monoacylglycerol Lipase Inhibitors: Discovery of a New Class of Benzylpiperidine Derivatives
- PMID: 35522977
- PMCID: PMC9150076
- DOI: 10.1021/acs.jmedchem.1c01806
Reversible Monoacylglycerol Lipase Inhibitors: Discovery of a New Class of Benzylpiperidine Derivatives
Abstract
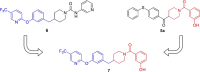
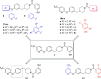
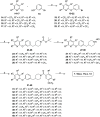
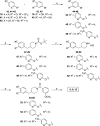
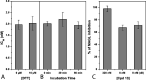
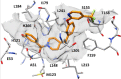
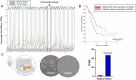
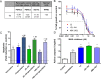
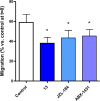
Monoacylglycerol lipase (MAGL) is the enzyme responsible for the metabolism of 2-arachidonoylglycerol in the brain and the hydrolysis of peripheral monoacylglycerols. Many studies demonstrated beneficial effects deriving from MAGL inhibition for neurodegenerative diseases, inflammatory pathologies, and cancer. MAGL expression is increased in invasive tumors, furnishing free fatty acids as pro-tumorigenic signals and for tumor cell growth. Here, a new class of benzylpiperidine-based MAGL inhibitors was synthesized, leading to the identification of 13, which showed potent reversible and selective MAGL inhibition. Associated with MAGL overexpression and the prognostic role in pancreatic cancer, derivative 13 showed antiproliferative activity and apoptosis induction, as well as the ability to reduce cell migration in primary pancreatic cancer cultures, and displayed a synergistic interaction with the chemotherapeutic drug gemcitabine. These results suggest that the class of benzylpiperidine-based MAGL inhibitors have potential as a new class of therapeutic agents and MAGL could play a role in pancreatic cancer.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Rajesh M.; Pan H.; Mukhopadhyay P.; Batkai S.; Osei-Hyiaman D.; Hasko G.; Liaudet L.; Gao B.; Pacher P. Pivotal Advance: Cannabinoid-2 Receptor Agonist HU-308 Protects against Hepatic Ischemia/Reperfusion Injury by Attenuating Oxidative Stress, Inflammatory Response, and Apoptosis. J. Leukocyte Biol. 2007, 82, 1382–1389. 10.1189/jlb.0307180. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical

