Priming of Arabidopsis resistance to herbivory by insect egg deposition depends on the plant's developmental stage
- PMID: 35522985
- PMCID: PMC9366327
- DOI: 10.1093/jxb/erac199
Priming of Arabidopsis resistance to herbivory by insect egg deposition depends on the plant's developmental stage
Abstract
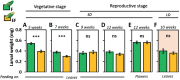
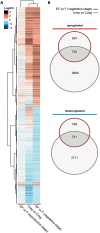
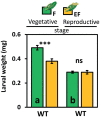
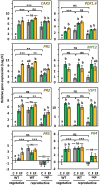
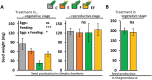
While traits of plant resistance to herbivory often change during ontogeny, it is unknown whether the primability of this resistance depends on the plant's developmental stage. Resistance in non-flowering Arabidopsis thaliana against Pieris brassicae larvae is known to be primable by prior egg deposition on leaves. We investigated whether this priming effect is maintained in plants at the flowering stage. Larval performance assays revealed that flowering plants' resistance to herbivory was not primable by egg deposition. Accordingly, transcriptomes of flowering plants showed almost no response to eggs. In contrast, egg deposition on non-flowering plants enhanced the expression of genes induced by subsequent larval feeding. Strikingly, flowering plants showed constitutively high expression levels of these genes. Larvae performed generally worse on flowering than on non-flowering plants, indicating that flowering plants constitutively resist herbivory. Furthermore, we determined the seed weight in regrown plants that had been exposed to eggs and larvae during the non-flowering or flowering stage. Non-flowering plants benefitted from egg priming with a smaller loss in seed yield. The seed yield of flowering plants was unaffected by the treatments, indicating tolerance towards the larvae. Our results show that the primability of anti-herbivore defences in Arabidopsis depends on the plant's developmental stage.
Keywords: herbivory; insect eggs; larval feeding; plant ontogeny; plant resistance; plant tolerance; priming; salicylic acid; seed yield; transcriptome.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures




References
-
- Agrawal AA. 1998. Induced responses to herbivory and increased plant performance. Science 279, 1201–1202. - PubMed
-
- Agrawal AA. 2000. Overcompensation of plants in response to herbivory and the by-product benefits of mutualism. Trends in Plant Science 5, 309–313. - PubMed
-
- Agrawal AA. 2011. Current trends in the evolutionary ecology of plant defence. Functional Ecology 25, 420–432.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

