Tonic pain alters functional connectivity of the descending pain modulatory network involving amygdala, periaqueductal gray, parabrachial nucleus and anterior cingulate cortex
- PMID: 35523367
- PMCID: PMC9250649
- DOI: 10.1016/j.neuroimage.2022.119278
Tonic pain alters functional connectivity of the descending pain modulatory network involving amygdala, periaqueductal gray, parabrachial nucleus and anterior cingulate cortex
Abstract
Introduction: Resting state functional connectivity (FC) is widely used to assess functional brain alterations in patients with chronic pain. However, reports of FC accompanying tonic pain in pain-free persons are rare. A network we term the Descending Pain Modulatory Network (DPMN) is implicated in healthy and pathologic pain modulation. Here, we evaluate the effect of tonic pain on FC of specific nodes of this network: anterior cingulate cortex (ACC), amygdala (AMYG), periaqueductal gray (PAG), and parabrachial nuclei (PBN).
Methods: In 50 pain-free participants (30F), we induced tonic pain using a capsaicin-heat pain model. functional MRI measured resting BOLD signal during pain-free rest with a 32 °C thermode and then tonic pain where participants experienced a previously warm temperature combined with capsaicin. We evaluated FC from ACC, AMYG, PAG, and PBN with correlation of self-report pain intensity during both states. We hypothesized tonic pain would diminish FC dyads within the DPMN.
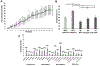
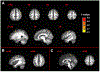
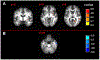
Results: Of all hypothesized FC dyads, only PAG and subgenual ACC was weakly altered during pain (F = 3.34; p = 0.074; pain-free>pain d = 0.25). After pain induction sACC-PAG FC became positively correlated with pain intensity (R = 0.38; t = 2.81; p = 0.007). Right PBN-PAG FC during pain-free rest positively correlated with subsequently experienced pain (R = 0.44; t = 3.43; p = 0.001). During pain, this connection's FC was diminished (paired t=-3.17; p = 0.0026). In whole-brain analyses, during pain-free rest, FC between left AMYG and right superior parietal lobule and caudate nucleus were positively correlated with subsequent pain. During pain, FC between left AMYG and right inferior temporal gyrus negatively correlated with pain. Subsequent pain positively correlated with right AMYG FC with right claustrum; right primary visual cortex and right temporo-occipitoparietal junction CONCLUSION: We demonstrate sACC-PAG tonic pain FC positively correlates with experienced pain and resting right PBN-PAG FC correlates with subsequent pain and is diminished during tonic pain. Finally, we reveal PAG- and right AMYG-anchored networks which correlate with subsequently experienced pain intensity. Our findings suggest specific connectivity patterns within the DPMN at rest are associated with subsequently experienced pain and modulated by tonic pain. These nodes and their functional modulation may reveal new therapeutic targets for neuromodulation or biomarkers to guide interventions.
Keywords: Capsaicin-heat pain model; Central sensitization; Descending pain modulatory network; Parabrachial nuclei; Periaqueductal gray; Seed-driven functional connectivity.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

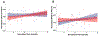
References
-
- An X, Bandler R, Ongur D, Price JL, 1998. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J. Comp. Neurol 401, 455–479. - PubMed
-
- Anderson WS, Sheth RN, Bencherif B, Frost JJ, Campbell JN, 2002. Naloxone increases pain induced by topical capsaicin in healthy human volunteers. Pain 99, 207–216. - PubMed
-
- Ayoub LJ, McAndrews MP, Barnett AJ, Jeremy Ho KC, Cioffi I, Moayedi M, 2021. Baseline resting-state functional connectivity determines subsequent pain ratings to a tonic ecologically valid experimental model of orofacial pain. Pain 162, 2397–2404 Articles in Press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

