Longitudinal T-Cell Responses After a Third SARS-CoV-2 Vaccination in Patients With Multiple Sclerosis on Ocrelizumab or Fingolimod
- PMID: 35523569
- PMCID: PMC9082763
- DOI: 10.1212/NXI.0000000000001178
Longitudinal T-Cell Responses After a Third SARS-CoV-2 Vaccination in Patients With Multiple Sclerosis on Ocrelizumab or Fingolimod
Erratum in
-
Longitudinal T-Cell Responses After a Third SARS-CoV-2 Vaccination in Patients With Multiple Sclerosis on Ocrelizumab or Fingolimod.Neurol Neuroimmunol Neuroinflamm. 2022 Aug 26;9(6):e200036. doi: 10.1212/NXI.0000000000200036. Print 2022 Nov. Neurol Neuroimmunol Neuroinflamm. 2022. PMID: 36028312 Free PMC article. No abstract available.
Abstract
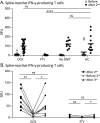
Objectives: To evaluate whether a third vaccination shows an added effect on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) T-cell responses in patients with multiple sclerosis treated with ocrelizumab or fingolimod.
Methods: This is a substudy of a prospective multicenter study on SARS-CoV-2 vaccination in patients with immune-mediated diseases. Patients with MS treated with ocrelizumab, fingolimod, and no disease-modifying therapies and healthy controls were included. The number of interferon (IFN)-γ secreting SARS-CoV-2-specific T cells at multiple time points before and after 3 SARS-CoV-2 vaccinations were evaluated.
Results: In ocrelizumab-treated patients (N = 24), IFN-γ-producing SARS-CoV-2-specific T-cell responses were induced after 2 vaccinations with median levels comparable to healthy controls (N = 12) and patients with MS without disease-modifying therapies (N = 10). A third vaccination in ocrelizumab-treated patients (N = 8) boosted T-cell responses that had declined after the second vaccination, but did not lead to higher overall T-cell responses as compared to immediately after a second vaccination. In fingolimod-treated patients, no SARS-CoV-2-specific T cells were detected after second (N = 12) and third (N = 9) vaccinations.
Discussion: In ocrelizumab-treated patients with MS, a third SARS-CoV-2 vaccination had no additive effect on the maximal T-cell response but did induce a boost response. In fingolimod-treated patients, no T-cell responses could be detected following both a second and third SARS-CoV-2 vaccination.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
-
- Mehling M, Hilbert P, Fritz S, et al. . Antigen-specific adaptive immune responses in fingolimod-treated multiple sclerosis patients. Ann Neurol. 2011;69(2):408-413. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
