Tumor Lysis Syndrome and AKI: Beyond Crystal Mechanisms
- PMID: 35523579
- PMCID: PMC9161807
- DOI: 10.1681/ASN.2021070997
Tumor Lysis Syndrome and AKI: Beyond Crystal Mechanisms
Abstract
Background: The pathophysiology of AKI during tumor lysis syndrome (TLS) is not well understood due to the paucity of data. We aimed to decipher crystal-dependent and crystal-independent mechanisms of TLS-induced AKI.
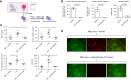
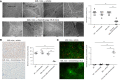
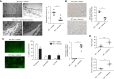
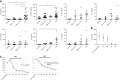
Methods: Crystalluria, plasma cytokine levels, and extracellular histones levels were measured in two cohorts of patients with TLS. We developed a model of TLS in syngeneic mice with acute myeloid leukemia, and analyzed ultrastructural changes in kidneys and endothelial permeability using intravital confocal microscopy. In parallel, we studied the endothelial toxicity of extracellular histones in vitro. RESULTS: The study provides the first evidence that previously described crystal-dependent mechanisms are insufficient to explain TLS-induced AKI. Extracellular histones that are released in huge amounts during TLS caused profound endothelial alterations in the mouse model. The mechanisms of histone-mediated damage implicates endothelial cell activation mediated by Toll-like receptor 4. Heparin inhibits extracellular histones and mitigates endothelial dysfunction during TLS.
Conclusion: This study sheds new light on the pathophysiology of TLS-induced AKI and suggests that extracellular histones may constitute a novel target for therapeutic intervention in TLS when endothelial dysfunction occurs.
Keywords: acute renal failure; endothelium; histones; tumor lysis syndrome.
Copyright © 2022 by the American Society of Nephrology.
Figures




Comment in
-
Crystals or His(stones): Rethinking AKI in Tumor Lysis Syndrome.J Am Soc Nephrol. 2022 Jun;33(6):1055-1057. doi: 10.1681/ASN.2022040425. J Am Soc Nephrol. 2022. PMID: 35641305 Free PMC article. No abstract available.
-
Understanding the Multiple Roles of Extracellular Histones in Mediating Endothelial Dysfunction.J Am Soc Nephrol. 2022 Oct;33(10):1951-1952. doi: 10.1681/ASN.2022060640. Epub 2022 Aug 3. J Am Soc Nephrol. 2022. PMID: 35922133 Free PMC article. No abstract available.
-
Authors' Reply: Understanding the Multiple Roles of Extracellular Histones in Mediating Endothelial Dysfunction.J Am Soc Nephrol. 2022 Oct;33(10):1952-1953. doi: 10.1681/ASN.2022060687. Epub 2022 Aug 3. J Am Soc Nephrol. 2022. PMID: 35922134 Free PMC article. No abstract available.
-
A Crystal-Independent Role for Uric Acid in AKI Associated with Tumor Lysis Syndrome.J Am Soc Nephrol. 2023 Jan 1;34(1):175. doi: 10.1681/ASN.0000000000000018. J Am Soc Nephrol. 2023. PMID: 36719148 Free PMC article. No abstract available.
-
Authors' Reply: A Crystal-Independent Role for Uric Acid in AKI Associated with Tumor Lysis Syndrome.J Am Soc Nephrol. 2023 Jan 1;34(1):176-177. doi: 10.1681/ASN.2022101097. J Am Soc Nephrol. 2023. PMID: 36719149 Free PMC article. No abstract available.
References
-
- Zafrani L, Canet E, Darmon M: Understanding tumor lysis syndrome. Intensive Care Med 45: 1608–1611, 2019 - PubMed
-
- Darmon M, Vincent F, Camous L, Canet E, Bonmati C, Braun T, et al. ; Groupe de Recherche en Réanimation Respiratoire et Onco-Hématologique (GRRR-OH) : Tumour lysis syndrome and acute kidney injury in high-risk haematology patients in the rasburicase era. A prospective multicentre study from the Groupe de Recherche en Réanimation Respiratoire et Onco-Hématologique. Br J Haematol 162: 489–497, 2013 - PubMed
-
- Boles JM, Dutel JL, Briere J, Mialon P, Robasckiewicz M, Garre M, et al. : Acute renal failure caused by extreme hyperphosphatemia after chemotherapy of an acute lymphoblastic leukemia. Cancer 53: 2425–2429, 1984 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

