Glutamate Transporters EAAT2 and EAAT5 Differentially Shape Synaptic Transmission from Rod Bipolar Cell Terminals
- PMID: 35523583
- PMCID: PMC9121915
- DOI: 10.1523/ENEURO.0074-22.2022
Glutamate Transporters EAAT2 and EAAT5 Differentially Shape Synaptic Transmission from Rod Bipolar Cell Terminals
Abstract
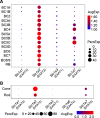
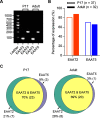
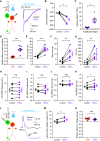
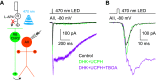
Excitatory amino acid transporters (EAATs) control visual signal transmission in the retina by rapidly removing glutamate released from photoreceptors and bipolar cells (BCs). Although it has been reported that EAAT2 and EAAT5 are expressed at presynaptic terminals of photoreceptors and some BCs in mammals, the distinct functions of these two glutamate transporters in retinal synaptic transmission, especially at a single synapse, remain elusive. In this study, we found that EAAT2 was expressed in all BC types while coexisting with EAAT5 in rod bipolar (RB) cells and several types of cone BCs from mice of either sex. Our immunohistochemical study, together with a recently published literature (Gehlen et al., 2021), showed that EAAT2 and EAAT5 were both located in RB axon terminals near release sites. Optogenetic, electrophysiological and pharmacological analyses, however, demonstrated that EAAT2 and EAAT5 regulated neurotransmission at RB→AII amacrine cell synapses in significantly different ways: EAAT5 dramatically affected both the peak amplitude and kinetics of postsynaptic responses in AIIs, whereas EAAT2 had either relatively small or opposite effects. By contrast, blockade of EAAT1/GLAST, which was exclusively expressed in Müller cells, showed no obvious effect on AII responses, indicating that glutamate uptake by Müller cells did not influence synaptic transmission from RB terminals. Furthermore, we found that temporal resolution at RB→AII synapses was reduced substantially by blockade of EAAT5 but not EAAT2. Taken together, our work reveals the distinct functions of EAAT2 and EAAT5 in signal transmission at RB ribbon synapses.
Keywords: amacrine cell; bipolar cell; glutamate transporter; retina; synaptic transmission; temporal resolution.
Copyright © 2022 Tang et al.
Figures



References
-
- Barnett NL, Pow DV (2000) Antisense knockdown of GLAST, a glial glutamate transporter, compromises retinal function. Invest Ophthalmol Vis Sci 41:585–591. - PubMed
-
- Blondel VD, Guillaume JL, Lambiotte R, Lefebvre E (2008) Fast unfolding of communities in large networks. J Stat Mech 2008:P10008. 10.1088/1742-5468/2008/10/P10008 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
