N-terminal alterations turn the gut hormone GLP-2 into an antagonist with gradual loss of GLP-2 receptor selectivity towards more GLP-1 receptor interaction
- PMID: 35523760
- PMCID: PMC9541843
- DOI: 10.1111/bph.15866
N-terminal alterations turn the gut hormone GLP-2 into an antagonist with gradual loss of GLP-2 receptor selectivity towards more GLP-1 receptor interaction
Abstract
Background and purpose: To fully elucidate the regulatory role of the GLP-2 system in the gut and the bones, potent and selective GLP-2 receptor (GLP-2R) antagonists are needed. Searching for antagonist activity, we performed systematic N-terminal truncations of human GLP-2(1-33).
Experimental approach: COS-7 cells were transfected with the human GLP-2R and assessed for cAMP accumulation or competition binding using 125 I-GLP-2(1-33)[M10Y]. To examine selectivity, COS-7 cells expressing human GLP-1 or GIP receptors were assessed for cAMP accumulation.
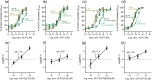
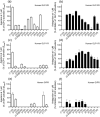
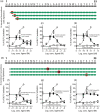
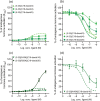
Key results: Affinity of the N-terminally truncated GLP-2 peptides for the GLP-2 receptor decreased with reduced N-terminal peptide length (Ki 6.5-871 nM), while increasing antagonism appeared with inhibitory potencies (IC50 ) values from 79 to 204 nM for truncation up to GLP-2(4-33) and then declined. In contrast, truncation-dependent increases in intrinsic activity were observed from an Emax of only 20% for GLP-(2-33) up to 46% for GLP-2(6-33) at 1 μM, followed by a decline. GLP-2(9-33) had the highest intrinsic efficacy (Emax 65%) and no antagonistic properties. Moreover, with truncations up to GLP-2(8-33), a gradual loss in selectivity for the GLP-2 receptor appeared with increasing GLP-1 receptor (GLP-1R) inhibition (up to 73% at 1 μM). Lipidation of the peptides improved antagonism (IC50 down to 7.9 nM) for both the GLP-2 and the GLP-1R.
Conclusion and implications: The N-terminus of GLP-2 is crucial for GLP-2R activity and selectivity. Our observations form the basis for the development of tool compounds for further characterization of the GLP-2 system.
Keywords: GLP-1 receptor; GLP-2; GLP-2 receptor; GPCR; N-terminus; antagonists; family B1 GPCR.
© 2022 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. MBNG, LSG, JJH and MMR are co‐founders of Antag Therapeutics ApS. MMR and JJH are also co‐founders of Bainan Biotech ApS.
Figures



Similar articles
-
N-terminally and C-terminally truncated forms of glucose-dependent insulinotropic polypeptide are high-affinity competitive antagonists of the human GIP receptor.Br J Pharmacol. 2016 Mar;173(5):826-38. doi: 10.1111/bph.13384. Epub 2016 Jan 30. Br J Pharmacol. 2016. PMID: 26572091 Free PMC article.
-
Novel agonist and antagonist radioligands for the GLP-2 receptor. Useful tools for studies of basic GLP-2 receptor pharmacology.Br J Pharmacol. 2022 May;179(9):1998-2015. doi: 10.1111/bph.15766. Epub 2022 Jan 11. Br J Pharmacol. 2022. PMID: 34855984 Free PMC article.
-
Differential Responses of the GLP-1 and GLP-2 Receptors to N-Terminal Modification of a Dual Agonist.J Am Chem Soc. 2023 Jun 7;145(22):12105-12114. doi: 10.1021/jacs.3c01628. Epub 2023 May 26. J Am Chem Soc. 2023. PMID: 37235770 Free PMC article.
-
Molecular interactions of full-length and truncated GIP peptides with the GIP receptor - A comprehensive review.Peptides. 2020 Mar;125:170224. doi: 10.1016/j.peptides.2019.170224. Epub 2019 Dec 3. Peptides. 2020. PMID: 31809770 Review.
-
GLP-1 and GIP receptor signaling in beta cells - A review of receptor interactions and co-stimulation.Peptides. 2022 May;151:170749. doi: 10.1016/j.peptides.2022.170749. Epub 2022 Jan 19. Peptides. 2022. PMID: 35065096 Review.
Cited by
-
Gut-Brain Axis Modulation of Metabolic Disorders: Exploring the Intertwined Neurohumoral Pathways and Therapeutic Prospects.Neurochem Res. 2024 Apr;49(4):847-871. doi: 10.1007/s11064-023-04084-7. Epub 2024 Jan 20. Neurochem Res. 2024. PMID: 38244132 Review.
-
Physiological functions of glucose transporter-2: From cell physiology to links with diabetes mellitus.Heliyon. 2024 Jan 29;10(3):e25459. doi: 10.1016/j.heliyon.2024.e25459. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38333863 Free PMC article. Review.
-
Comparative Effects of GLP-1 and GLP-2 on Beta-Cell Function, Glucose Homeostasis and Appetite Regulation.Biomolecules. 2024 Nov 27;14(12):1520. doi: 10.3390/biom14121520. Biomolecules. 2024. PMID: 39766228 Free PMC article.
References
-
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Boison, D. , Burns, K. E. , Dessauer, C. , Gertsch, J. , Helsby, N. A. , Izzo, A. A. , Koesling, D. , … Wong, S. S. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Enzymes. British Journal of Pharmacology, 178(S1), S313–S411. 10.1111/bph.15542 - DOI - PubMed
-
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Abbracchio, M. P. , Alexander, W. , al‐Hosaini, K. , Bäck, M. , Barnes, N. M. , Bathgate, R. , … Ye, R. D. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: G protein‐coupled receptors. British Journal of Pharmacology, 178(S1), S27–S156. 10.1111/bph.15538 - DOI - PubMed
-
- Askov‐Hansen, C. , Jeppesen, P. B. , Lund, P. , Hartmann, B. , Holst, J. J. , & Henriksen, D. B. (2013). Effect of glucagon‐like peptide‐2 exposure on bone resorption: Effectiveness of high concentration versus prolonged exposure. Regulatory Peptides, 181, 4–8. 10.1016/j.regpep.2012.11.002 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

