Transmembrane stem cell factor protein therapeutics enhance revascularization in ischemia without mast cell activation
- PMID: 35523773
- PMCID: PMC9076913
- DOI: 10.1038/s41467-022-30103-2
Transmembrane stem cell factor protein therapeutics enhance revascularization in ischemia without mast cell activation
Abstract
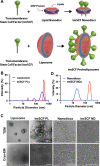
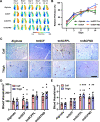
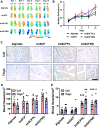
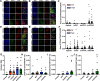
Stem cell factor (SCF) is a cytokine that regulates hematopoiesis and other biological processes. While clinical treatments using SCF would be highly beneficial, these have been limited by toxicity related to mast cell activation. Transmembrane SCF (tmSCF) has differential activity from soluble SCF and has not been explored as a therapeutic agent. We created novel therapeutics using tmSCF embedded in proteoliposomes or lipid nanodiscs. Mouse models of anaphylaxis and ischemia revealed the tmSCF-based therapies did not activate mast cells and improved the revascularization in the ischemic hind limb. Proteoliposomal tmSCF preferentially acted on endothelial cells to induce angiogenesis while tmSCF nanodiscs had greater activity in inducing stem cell mobilization and recruitment to the site of injury. The type of lipid nanocarrier used altered the relative cellular uptake pathways and signaling in a cell type dependent manner. Overall, we found that tmSCF-based therapies can provide therapeutic benefits without off target effects.
© 2022. The Author(s).
Conflict of interest statement
E.T. and A.B.B. have applied for a patent on the technology described in the paper (US20210145931A1).
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

