A serotonergic biobehavioral signature differentiates cocaine use disorder participants administered mirtazapine
- PMID: 35523779
- PMCID: PMC9076859
- DOI: 10.1038/s41398-022-01934-w
A serotonergic biobehavioral signature differentiates cocaine use disorder participants administered mirtazapine
Abstract
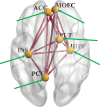
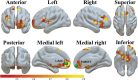
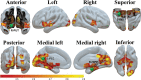
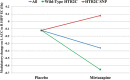
Cocaine use disorder (CUD) patients display heterogenous symptoms and unforeseeable responses to available treatment approaches, highlighting the need to identify objective, accessible biobehavioral signatures to predict clinical trial success in this population. In the present experiments, we employed a task-based behavioral and pharmacogenetic-fMRI approach to address this gap. Craving, an intense desire to take cocaine, can be evoked by exposure to cocaine-associated stimuli which can trigger relapse during attempted recovery. Attentional bias towards cocaine-associated words is linked to enhanced effective connectivity (EC) from the anterior cingulate cortex (ACC) to hippocampus in CUD participants, an observation which was replicated in a new cohort of participants in the present studies. Serotonin regulates attentional bias to cocaine and the serotonergic antagonist mirtazapine decreased activated EC associated with attentional bias, with greater effectiveness in those CUD participants carrying the wild-type 5-HT2CR gene relative to a 5-HT2CR single nucleotide polymorphism (rs6318). These data suggest that the wild-type 5-HT2CR is necessary for the efficacy of mirtazapine to decrease activated EC in CUD participants and that mirtazapine may serve as an abstinence enhancer to mitigate brain substrates of craving in response to cocaine-associated stimuli in participants with this pharmacogenetic descriptor. These results are distinctive in outlining a richer "fingerprint" of the complex neurocircuitry, behavior and pharmacogenetics profile of CUD participants which may provide insight into success of future medications development projects.
© 2022. The Author(s).
Conflict of interest statement
FGM has past research funding from Indivior Pharmaceuticals and Nektar Therapeutics for research unrelated to this study. KAC and NCA have current research funding from VidaLibreBio, Inc., for research unrelated to this study. Other authors declare no competing interests.
Figures
References
-
- NSDUH. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. (September 4, 2014). The NSDUH Report: Substance Use and Mental Health Estimates from the 2013 National Survey on Drug Use and Health: Overview of Findings. Rockville, MD: NSDUH, 2014. - PubMed
-
- Schmitz JM, Green CE, Stotts AL, Lindsay JA, Rathnayaka NS, Grabowski J, et al. A two-phased screening paradigm for evaluating candidate medications for cocaine cessation or relapse prevention: modafinil, levodopa-carbidopa, naltrexone. Drug Alcohol Depend. 2014;136:100–7. doi: 10.1016/j.drugalcdep.2013.12.015. - DOI - PMC - PubMed
-
- McCann DJ, Ramey T, Skolnick P. Outcome measures in medication trials for substance use disorders. Curr Treat Options Psychiatry. 2015;2:113–21. doi: 10.1007/s40501-015-0038-5. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

