Ataluren binds to multiple protein synthesis apparatus sites and competitively inhibits release factor-dependent termination
- PMID: 35523781
- PMCID: PMC9076611
- DOI: 10.1038/s41467-022-30080-6
Ataluren binds to multiple protein synthesis apparatus sites and competitively inhibits release factor-dependent termination
Abstract
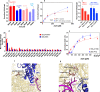
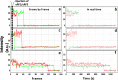
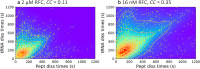
Genetic diseases are often caused by nonsense mutations, but only one TRID (translation readthrough inducing drug), ataluren, has been approved for clinical use. Ataluren inhibits release factor complex (RFC) termination activity, while not affecting productive binding of near-cognate ternary complex (TC, aa-tRNA.eEF1A.GTP). Here we use photoaffinity labeling to identify two sites of ataluren binding within rRNA, proximal to the decoding center (DC) and the peptidyl transfer center (PTC) of the ribosome, which are directly responsible for ataluren inhibition of termination activity. A third site, within the RFC, has as yet unclear functional consequences. Using single molecule and ensemble fluorescence assays we also demonstrate that termination proceeds via rapid RFC-dependent hydrolysis of peptidyl-tRNA followed by slow release of peptide and tRNA from the ribosome. Ataluren is an apparent competitive inhibitor of productive RFC binding, acting at or before the hydrolysis step. We propose that designing more potent TRIDs which retain ataluren's low toxicity should target areas of the RFC binding site proximal to the DC and PTC which do not overlap the TC binding site.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Mechanism-based approach in designing patient-specific combination therapies for nonsense mutation diseases.Nucleic Acids Res. 2025 Mar 20;53(6):gkaf216. doi: 10.1093/nar/gkaf216. Nucleic Acids Res. 2025. PMID: 40156864 Free PMC article.
-
A High-Throughput Assay for In Vitro Determination of Release Factor-Dependent Peptide Release from a Pretermination Complex by Fluorescence Anisotropy-Application to Nonsense Suppressor Screening and Mechanistic Studies.Biomolecules. 2023 Jan 27;13(2):242. doi: 10.3390/biom13020242. Biomolecules. 2023. PMID: 36830611 Free PMC article.
-
Ataluren and aminoglycosides stimulate read-through of nonsense codons by orthogonal mechanisms.Proc Natl Acad Sci U S A. 2021 Jan 12;118(2):e2020599118. doi: 10.1073/pnas.2020599118. Proc Natl Acad Sci U S A. 2021. PMID: 33414181 Free PMC article.
-
Strategies against Nonsense: Oxadiazoles as Translational Readthrough-Inducing Drugs (TRIDs).Int J Mol Sci. 2019 Jul 6;20(13):3329. doi: 10.3390/ijms20133329. Int J Mol Sci. 2019. PMID: 31284579 Free PMC article. Review.
-
The ribosome as a versatile catalyst: reactions at the peptidyl transferase center.Curr Opin Struct Biol. 2013 Aug;23(4):595-602. doi: 10.1016/j.sbi.2013.04.012. Epub 2013 May 24. Curr Opin Struct Biol. 2013. PMID: 23711800 Review.
Cited by
-
Boric acid intercepts 80S ribosome migration from AUG-stop by stabilizing eRF1.Nat Chem Biol. 2024 May;20(5):605-614. doi: 10.1038/s41589-023-01513-0. Epub 2024 Jan 24. Nat Chem Biol. 2024. PMID: 38267667
-
Pharmaceuticals Promoting Premature Termination Codon Readthrough: Progress in Development.Biomolecules. 2023 Jun 14;13(6):988. doi: 10.3390/biom13060988. Biomolecules. 2023. PMID: 37371567 Free PMC article. Review.
-
Readthrough Approach Using NV Translational Readthrough-Inducing Drugs (TRIDs): A Study of the Possible Off-Target Effects on Natural Termination Codons (NTCs) on TP53 and Housekeeping Gene Expression.Int J Mol Sci. 2023 Oct 11;24(20):15084. doi: 10.3390/ijms242015084. Int J Mol Sci. 2023. PMID: 37894764 Free PMC article.
-
Mechanism-based approach in designing patient-specific combination therapies for nonsense mutation diseases.Nucleic Acids Res. 2025 Mar 20;53(6):gkaf216. doi: 10.1093/nar/gkaf216. Nucleic Acids Res. 2025. PMID: 40156864 Free PMC article.
-
RNA as an off-target for FDA-approved drugs.Nat Chem. 2023 Oct;15(10):1329-1331. doi: 10.1038/s41557-023-01330-x. Nat Chem. 2023. PMID: 37697037 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

