The role of mitochondrial fission in cardiovascular health and disease
- PMID: 35523864
- PMCID: PMC10584015
- DOI: 10.1038/s41569-022-00703-y
The role of mitochondrial fission in cardiovascular health and disease
Abstract
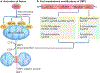
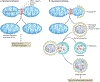
Mitochondria are organelles involved in the regulation of various important cellular processes, ranging from ATP generation to immune activation. A healthy mitochondrial network is essential for cardiovascular function and adaptation to pathological stressors. Mitochondria undergo fission or fusion in response to various environmental cues, and these dynamic changes are vital for mitochondrial function and health. In particular, mitochondrial fission is closely coordinated with the cell cycle and is linked to changes in mitochondrial respiration and membrane permeability. Another key function of fission is the segregation of damaged mitochondrial components for degradation by mitochondrial autophagy (mitophagy). Mitochondrial fission is induced by the large GTPase dynamin-related protein 1 (DRP1) and is subject to sophisticated regulation. Activation requires various post-translational modifications of DRP1, actin polymerization and the involvement of other organelles such as the endoplasmic reticulum, Golgi apparatus and lysosomes. A decrease in mitochondrial fusion can also shift the balance towards mitochondrial fission. Although mitochondrial fission is necessary for cellular homeostasis, this process is often aberrantly activated in cardiovascular disease. Indeed, strong evidence exists that abnormal mitochondrial fission directly contributes to disease development. In this Review, we compare the physiological and pathophysiological roles of mitochondrial fission and discuss the therapeutic potential of preventing excessive mitochondrial fission in the heart and vasculature.
© 2022. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures


References
-
- Frank S. et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Developmental cell 1, 515–525 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

