Achievement of complete in vitro spermatogenesis in testicular tissues from prepubertal mice exposed to mono- or polychemotherapy
- PMID: 35523907
- PMCID: PMC9076692
- DOI: 10.1038/s41598-022-11286-6
Achievement of complete in vitro spermatogenesis in testicular tissues from prepubertal mice exposed to mono- or polychemotherapy
Abstract
The assessment of the impact of chemotherapies on in vitro spermatogenesis in experimental models is required before considering the application of this fertility restoration strategy to prepubertal boys who received these treatments before testicular tissue cryopreservation. The present work investigated the effects of exposure of prepubertal mice to mono- (vincristine or cyclophosphamide) and polychemotherapy (a combination of vincristine and cyclophosphamide) on the first wave of in vitro spermatogenesis. When testicular tissue exposed to monochemotherapy was preserved, polychemotherapy led to severe alterations of the seminiferous epithelium and increased apoptosis in prepubertal testes prior in vitro maturation, suggesting a potential additive gonadotoxic effect. These alterations were also found in the testicular tissues of polychemotherapy-treated mice after 30 days of organotypic culture and were associated with a reduction in the germ cell/Sertoli cell ratio. The different treatments neither altered the ability of spermatogonia to differentiate in vitro into spermatozoa nor the yield of in vitro spermatogenesis. However, more spermatozoa with morphological abnormalities and fragmented DNA were produced after administration of polychemotherapy. This work therefore shows for the first time the possibility to achieve a complete in vitro spermatogenesis after an in vivo exposure of mice to a mono- or polychemotherapy before meiotic entry.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
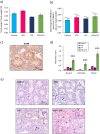
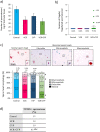
 ) and vacuoles (*). Data are expressed as means ± SEM with n = 4 mice for all groups. A value of *P < 0.05 was considered statistically significant. aP < 0.05 compared to control; bP < 0.05 compared to VCR and cP < 0.05 compared to CYP.
) and vacuoles (*). Data are expressed as means ± SEM with n = 4 mice for all groups. A value of *P < 0.05 was considered statistically significant. aP < 0.05 compared to control; bP < 0.05 compared to VCR and cP < 0.05 compared to CYP.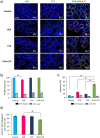
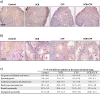
 ). (c) Percentage of seminiferous tubules at the most advanced differentiation stage of spermatogenesis after in vitro maturation of testicular tissues from mice exposed to VCR, CYP, VCR+CYP or unexposed (control) during the prepubertal period. Data are presented as means ± SEM with n = 4 testicular explants for each group. A value of *P < 0.05 was considered statistically significant.
). (c) Percentage of seminiferous tubules at the most advanced differentiation stage of spermatogenesis after in vitro maturation of testicular tissues from mice exposed to VCR, CYP, VCR+CYP or unexposed (control) during the prepubertal period. Data are presented as means ± SEM with n = 4 testicular explants for each group. A value of *P < 0.05 was considered statistically significant.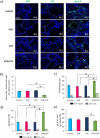
Similar articles
-
In vivo and in vitro spermatogenesis in prepubertal mouse testes exposed to low gonadotoxic doses of cytarabine or Daunorubicin.Sci Rep. 2025 Apr 24;15(1):14230. doi: 10.1038/s41598-025-98413-1. Sci Rep. 2025. PMID: 40275009 Free PMC article.
-
Evaluation of apoptotic- and autophagic-related protein expressions before and after IVM of fresh, slow-frozen and vitrified pre-pubertal mouse testicular tissue.Mol Hum Reprod. 2017 Nov 1;23(11):738-754. doi: 10.1093/molehr/gax054. Mol Hum Reprod. 2017. PMID: 29040674
-
Impact of low- or moderate-risk gonadotoxic chemotherapy prior to testicular tissue freezing on spermatogonia quantity in human (pre)pubertal testicular tissue.Hum Reprod. 2023 Nov 2;38(11):2105-2118. doi: 10.1093/humrep/dead161. Hum Reprod. 2023. PMID: 37674325
-
Spermatogenesis by Sisyphus: proliferating stem germ cells fail to repopulate the testis after 'irreversible' injury.Adv Exp Med Biol. 2001;500:421-8. doi: 10.1007/978-1-4615-0667-6_64. Adv Exp Med Biol. 2001. PMID: 11764975 Review.
-
Tissue Engineering to Improve Immature Testicular Tissue and Cell Transplantation Outcomes: One Step Closer to Fertility Restoration for Prepubertal Boys Exposed to Gonadotoxic Treatments.Int J Mol Sci. 2018 Jan 18;19(1):286. doi: 10.3390/ijms19010286. Int J Mol Sci. 2018. PMID: 29346308 Free PMC article. Review.
Cited by
-
Differentiation Disorders of Chara vulgaris Spermatids following Treatment with Propyzamide.Cells. 2023 Apr 27;12(9):1268. doi: 10.3390/cells12091268. Cells. 2023. PMID: 37174667 Free PMC article.
-
In vivo and in vitro spermatogenesis in prepubertal mouse testes exposed to low gonadotoxic doses of cytarabine or Daunorubicin.Sci Rep. 2025 Apr 24;15(1):14230. doi: 10.1038/s41598-025-98413-1. Sci Rep. 2025. PMID: 40275009 Free PMC article.
-
Male infertility.Nat Rev Dis Primers. 2023 Sep 14;9(1):49. doi: 10.1038/s41572-023-00459-w. Nat Rev Dis Primers. 2023. PMID: 37709866 Review.
-
Alkylating agents-induced gonadotoxicity in prepubertal males: Insights on the clinical and preclinical front.Clin Transl Sci. 2024 Jul;17(7):e13866. doi: 10.1111/cts.13866. Clin Transl Sci. 2024. PMID: 38965809 Free PMC article. Review.
References
-
- Brougham MFH, Wallace WHB. Subfertility in children and young people treated for solid and haematological malignancies. Br. J. Haematol. 2005;131:143–155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

