Interferon blockade in lupus: effects on antiviral immunity
- PMID: 35523957
- PMCID: PMC9073448
- DOI: 10.1038/s41581-022-00581-0
Interferon blockade in lupus: effects on antiviral immunity
Abstract
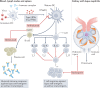
Targeting type I interferon immune responses is a potential strategy for the treatment of systemic lupus erythematosus. Although a phase 2 clinical trial of anifrolumab did not meet its primary end point, further studies are needed to assess the effects of interferon blockade on flare rates of lupus nephritis. However, the observed higher risk of herpes zoster associated with anifrolumab use suggests that caution is warranted with this strategy.
Conflict of interest statement
H.-J.A. has received consultancy fees from Bayer, Janssen, GSK, Novartis, Boehringer, AstraZeneca and PreviPharma. S.S. has received research funding from Eleva Ltd.
Figures
Comment on
-
Phase II randomised trial of type I interferon inhibitor anifrolumab in patients with active lupus nephritis.Ann Rheum Dis. 2022 Apr;81(4):496-506. doi: 10.1136/annrheumdis-2021-221478. Epub 2022 Feb 10. Ann Rheum Dis. 2022. PMID: 35144924 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

