Disorders of ubiquitylation: unchained inflammation
- PMID: 35523963
- PMCID: PMC9075716
- DOI: 10.1038/s41584-022-00778-4
Disorders of ubiquitylation: unchained inflammation
Abstract
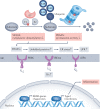
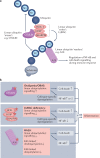
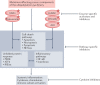
Ubiquitylation is an essential post-translational modification that regulates intracellular signalling networks by triggering proteasomal substrate degradation, changing the activity of substrates or mediating changes in proteins that interact with substrates. Hundreds of enzymes participate in reversible ubiquitylation of proteins, some acting globally and others targeting specific proteins. Ubiquitylation is essential for innate immune responses, as it facilitates rapid regulation of inflammatory pathways, thereby ensuring sufficient but not excessive responses. A growing number of inborn errors of immunity are attributed to dysregulated ubiquitylation. These genetic disorders exhibit broad clinical manifestations, ranging from susceptibility to infection to autoinflammatory and/or autoimmune features, lymphoproliferation and propensity to malignancy. Many autoinflammatory disorders result from disruption of components of the ubiquitylation machinery and lead to overactivation of innate immune cells. An understanding of the disorders of ubiquitylation in autoinflammatory diseases could enable the development of novel management strategies.
© 2022. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

