Trans-Seq maps a selective mammalian retinotectal synapse instructed by Nephronectin
- PMID: 35524141
- PMCID: PMC9172271
- DOI: 10.1038/s41593-022-01068-8
Trans-Seq maps a selective mammalian retinotectal synapse instructed by Nephronectin
Abstract
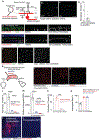
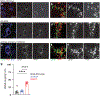
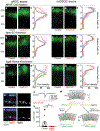
The mouse visual system serves as an accessible model to understand mammalian circuit wiring. Despite rich knowledge in retinal circuits, the long-range connectivity map from distinct retinal ganglion cell (RGC) types to diverse brain neuron types remains unknown. In this study, we developed an integrated approach, called Trans-Seq, to map RGCs to superior collicular (SC) circuits. Trans-Seq combines a fluorescent anterograde trans-synaptic tracer, consisting of codon-optimized wheat germ agglutinin fused to mCherry, with single-cell RNA sequencing. We used Trans-Seq to classify SC neuron types innervated by genetically defined RGC types and predicted a neuronal pair from αRGCs to Nephronectin-positive wide-field neurons (NPWFs). We validated this connection using genetic labeling, electrophysiology and retrograde tracing. We then used transcriptomic data from Trans-Seq to identify Nephronectin as a determinant for selective synaptic choice from αRGC to NPWFs via binding to Integrin α8β1. The Trans-Seq approach can be broadly applied for post-synaptic circuit discovery from genetically defined pre-synaptic neurons.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Figures















References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

