Three birds with one stone: co-encapsulation of diclofenac and DL-menthol for realizing enhanced energy deposition, glycolysis inhibition and anti-inflammation in HIFU surgery
- PMID: 35524259
- PMCID: PMC9074192
- DOI: 10.1186/s12951-022-01437-2
Three birds with one stone: co-encapsulation of diclofenac and DL-menthol for realizing enhanced energy deposition, glycolysis inhibition and anti-inflammation in HIFU surgery
Abstract
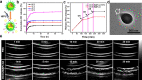
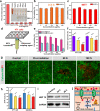
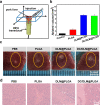
Despite attracting increasing attention in clinic, non-invasive high-intensity focused ultrasound (HIFU) surgery still commonly suffers from tumor recurrence and even matastasis due to the generation of thermo-resistance in non-apoptotic tumor cells and adverse therapy-induced inflammation with enhanced secretion of growth factors in irradiated region. In this work, inspired by the intrinsic property that the expression of thermo-resistant heat shock proteins (HSPs) is highly dependent with adenosine triphosphate (ATP), dual-functionalized diclofenac (DC) with anti-inflammation and glycolysis-inhibition abilities was successfully co-encapsulated with phase-change dl-menthol (DLM) in poly(lactic-co-glycolic acid) nanoparticles (DC/DLM@PLGA NPs) to realize improved HIFU surgery without causing adverse inflammation. Both in vitro and in vivo studies demonstrated the great potential of DC/DLM@PLGA NPs for serving as an efficient synergistic agent for HIFU surgery, which can not only amplify HIFU ablation efficacy through DLM vaporization-induced energy deposition but also simultaneously sensitize tumor cells to hyperthermia by glycolysis inhibition as well as diminished inflammation. Thus, our study provides an efficient strategy for simultaneously improving the curative efficiency and diminishing the harmful inflammatory responses of clinical HIFU surgery.
Keywords: Anti-inflammation; Diclofenac; Glycolysis inhibition; High-intensity focused ultrasound; Phase-change.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
dl-Menthol Loaded Polypyrrole Nanoparticles as a Controlled Diclofenac Delivery Platform for Sensitizing Cancer Cells to Photothermal Therapy.ACS Appl Bio Mater. 2019 Feb 18;2(2):848-855. doi: 10.1021/acsabm.8b00687. Epub 2019 Feb 1. ACS Appl Bio Mater. 2019. PMID: 35016288
-
Methotrexate-loaded PLGA nanobubbles for ultrasound imaging and Synergistic Targeted therapy of residual tumor during HIFU ablation.Biomaterials. 2014 Jun;35(19):5148-61. doi: 10.1016/j.biomaterials.2014.02.036. Epub 2014 Mar 28. Biomaterials. 2014. PMID: 24680663
-
Polyethylenimine (PEI)-modified poly (lactic-co-glycolic) acid (PLGA) nanoparticles conjugated with tumor-homing bacteria facilitate high intensity focused ultrasound-mediated tumor ablation.Biochem Biophys Res Commun. 2021 Sep 24;571:104-109. doi: 10.1016/j.bbrc.2021.07.061. Epub 2021 Jul 24. Biochem Biophys Res Commun. 2021. PMID: 34314995
-
Phase-Changeable Nanoparticle-Mediated Energy Conversion Promotes Highly Efficient High-Intensity Focused Ultrasound Ablation.Curr Med Chem. 2022 Mar 4;29(8):1369-1378. doi: 10.2174/0929867328666210708085110. Curr Med Chem. 2022. PMID: 34238143 Review.
-
High-intensity focused ultrasound (HIFU) ablation versus surgical interventions for the treatment of symptomatic uterine fibroids: a meta-analysis.Eur Radiol. 2022 Feb;32(2):1195-1204. doi: 10.1007/s00330-021-08156-6. Epub 2021 Aug 1. Eur Radiol. 2022. PMID: 34333684 Review.
Cited by
-
Application of high intensity focused ultrasound combined with nanomaterials in anti-tumor therapy.Drug Deliv. 2024 Dec;31(1):2342844. doi: 10.1080/10717544.2024.2342844. Epub 2024 Apr 24. Drug Deliv. 2024. PMID: 38659328 Free PMC article. Review.
-
Synergistic metabolic modulation of fibroblast-like synoviocytes via targeted dual prodrug nanoparticles to mitigate rheumatoid arthritis.Acta Pharm Sin B. 2025 Jan;15(1):542-556. doi: 10.1016/j.apsb.2024.11.007. Epub 2024 Nov 18. Acta Pharm Sin B. 2025. PMID: 40041914 Free PMC article.
-
Shikonin-Loaded Hollow Fe-MOF Nanoparticles for Enhanced Microwave Thermal Therapy.ACS Biomater Sci Eng. 2023 Sep 11;9(9):5405-5417. doi: 10.1021/acsbiomaterials.3c00644. Epub 2023 Aug 28. ACS Biomater Sci Eng. 2023. PMID: 37638660 Free PMC article.
References
-
- Kennedy J. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5:321–7. - PubMed
-
- Illing R, Kennedy J, Wu F, Haar G, Protheroe A, Friend P, Gleeson F, Cranston D, Phillips R, Middleton M. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer. 2005;93:890–5. - PMC - PubMed
-
- Li C, Zhang W, Fan W, Huang J, Zhang F, Wu P. Noninvasive treatment of malignant bone tumors using high-intensity focused ultrasound. Cancer. 2010;116:3934–42. - PubMed
-
- Al-Bataineh O, Jenne J, Huber P. Clinical and future applications of high intensity focused ultrasound in cancer. Cancer Treat Rev. 2012;38:346–53. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

