Likelihood of response with subsequent dosing for patients with migraine and initial suboptimal response with eptinezumab: A post hoc analysis of two placebo-controlled randomized clinical trials
- PMID: 35524405
- PMCID: PMC9321567
- DOI: 10.1111/head.14302
Likelihood of response with subsequent dosing for patients with migraine and initial suboptimal response with eptinezumab: A post hoc analysis of two placebo-controlled randomized clinical trials
Abstract
Objective: To develop a multivariable model assessing factors predicting a second-dose response to eptinezumab treatment over weeks 13-24 in patients with migraine initially reporting a suboptimal response over weeks 1-12.
Background: Eptinezumab is a monoclonal antibody used for migraine prevention, administered every 12 weeks. In the PROMISE-1 and PROMISE-2 studies, the first-dose response to eptinezumab treatment (≥50% monthly migraine day [MMD] reduction over weeks 1-12) occurred in ~50-60% of patients with episodic (EM) and chronic migraine (CM), respectively.
Methods: This post hoc analysis included patients with suboptimal first-dose response (<50% MMD reduction over weeks 1-12) with EM and CM, with patient-reported outcome data at weeks 12 and 24. Eptinezumab 100 and 300 mg doses were pooled.
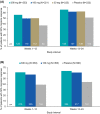
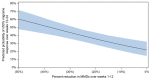
Results: The analysis included 416/888 patients (46.8%) from PROMISE-1 and 479/1072 patients (44.7%) from PROMISE-2 with suboptimal first-dose response. The proportion of suboptimal first-dose responders who were second-dose responders was 37.0% (71/192; eptinezumab) and 33.9% (42/124; placebo) in PROMISE-1 and 28.8% (79/274) and 18.5% (38/205) in PROMISE-2. Significant first-dose predictors of second-dose response were percent change in MMDs across weeks 1-12 (PROMISE-1, odds ratio [OR]: 0.97, 95% confidence interval [CI]: 0.95, 0.98, p = 0.0001; PROMISE-2, OR: 0.94, CI: 0.92, 0.96, p < 0.0001) and change in 6-item Headache Impact Test (HIT-6) total score (PROMISE-2 only, OR: 0.92; CI: 0.87, 0.98; p = 0.027). In PROMISE-1, the probability of second-dose response ranged from 21.7% in patients with first-dose 0% MMD change to 56.0% in patients with first-dose 45% MMD reduction. In PROMISE-2, depending on HIT-6 total score, probability of second-dose response ranged from 5.9-12.1% in patients with first-dose 0% MMD change to 54.2%-72.3% in patients with first-dose 45.0% MMD reduction.
Conclusion: Individuals with migraine not experiencing ≥50% MMD response to their first dose of eptinezumab may benefit from a second dose.
Keywords: PROMISE-1; PROMISE-2; chronic migraine; episodic migraine; eptinezumab; response.
© 2022 The Authors. Headache: The Journal of Head and Face Pain published by Wiley Periodicals LLC on behalf of American Headache Society.
Conflict of interest statement
LM has served as a consultant, advisory board member, and/or speaker for Abbvie, AEVI, Alkermes, Allergan, Amgen, Aptinyx, Arbor, Axsome, AZTherapies, Bayer, Biogen, Biohaven, Bionomics, BlackThorn, CoLucid, Daiichi Sankyo, Dr. Reddy's, Eli Lilly, Esperion, Intarcia, Intra‐Cellular, Ironshore, Janssen, Labrys Biologic, Lundbeck, Mitsubishi, Mylan, NLS Pharma, Nektar, Nestle Pamlab, Neuralstem, Neurocrine, Novartis, Novo Nordisk, ObsEva, Otsuka, Palatin, Pfizer, Regeneron, Rhodes, Shionogi, Shire, Sunovion, Supernus, Takeda, TauRx, and Teva. JDS has served on advisory boards for Aeon, Allergan, Amgen, Biohaven, electroCore, Impel, Lilly, Lundbeck, Novartis, Promius, Revance, Teva, and Upsher‐Smith; and has received compensation for speaking from Allergan, Amgen, Biohaven, electroCore, Eli Lilly, Lundbeck, Novartis, Promius, Teva, and Upsher‐Smith. CA, EB, and AO are full‐time employees of H. Lundbeck A/S or one of its subsidiary companies. RC was an employee of Lundbeck or one of its subsidiary companies at the time of study and manuscript development. JH is an employee of Pacific Northwest Statistical Consulting, Inc., a contracted service provider of biostatistical resources for H. Lundbeck A/S.
Figures


References
-
- Ailani J, Burch RC, Robbins MS; Board of Directors of the American Headache Society . The American Headache Society Consensus Statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61:1021‐1039. - PubMed
-
- Leonardi M, Raggi A, Bussone G, D'Amico D. Health‐related quality of life, disability and severity of disease in patients with migraine attending to a specialty headache center. Headache. 2010;50:1576‐1586. - PubMed
-
- Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343‐349. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

