The development of extracellular vesicle markers for the fungal phytopathogen Colletotrichum higginsianum
- PMID: 35524440
- PMCID: PMC9077143
- DOI: 10.1002/jev2.12216
The development of extracellular vesicle markers for the fungal phytopathogen Colletotrichum higginsianum
Abstract
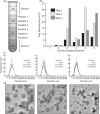
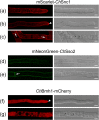
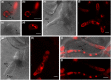
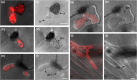
Fungal phytopathogens secrete extracellular vesicles (EVs) associated with enzymes and phytotoxic metabolites. While these vesicles are thought to promote infection, defining the true contents and functions of fungal EVs, as well as suitable protein markers, is an ongoing process. To expand our understanding of fungal EVs and their possible roles during infection, we purified EVs from the hemibiotrophic phytopathogen Colletotrichum higginsianum, the causative agent of anthracnose disease in multiple plant species, including Arabidopsis thaliana. EVs were purified in large numbers from the supernatant of protoplasts but not the supernatant of intact mycelial cultures. We purified two separate populations of EVs, each associated with over 700 detected proteins, including proteins involved in vesicle transport, cell wall biogenesis and the synthesis of secondary metabolites. We selected two SNARE proteins (Snc1 and Sso2) and one 14-3-3 protein (Bmh1) as potential EV markers and generated transgenic strains expressing fluorescent fusions. Each marker was confirmed to be protected inside EVs. Fluorescence microscopy was used to examine the localization of each marker during infection on Arabidopsis leaves. These findings further our understanding of EVs in fungal phytopathogens and will help build an experimental system to study EV interkingdom communication between plants and fungi.
Keywords: 14-3-3 proteins; Colletotrichum higginsianum; SNARE proteins; extracellular vesicles; phytopathogen; protoplasts.
© 2022 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors do not declare any conflicts of interest.
Figures







Similar articles
-
The dsRNA mycovirus ChNRV1 causes mild hypervirulence in the fungal phytopathogen Colletotrichum higginsianum.Arch Microbiol. 2021 Jan;203(1):241-249. doi: 10.1007/s00203-020-02030-7. Epub 2020 Sep 10. Arch Microbiol. 2021. PMID: 32914229
-
Dual Transcriptome Analysis Reveals That ChATG8 Is Required for Fungal Development, Melanization and Pathogenicity during the Interaction between Colletotrichum higginsianum and Arabidopsis thaliana.Int J Mol Sci. 2023 Feb 22;24(5):4376. doi: 10.3390/ijms24054376. Int J Mol Sci. 2023. PMID: 36901806 Free PMC article.
-
Arabidopsis Produces Distinct Subpopulations of Extracellular Vesicles That Respond Differentially to Biotic Stress, Altering Growth and Infectivity of a Fungal Pathogen.J Extracell Vesicles. 2025 May;14(5):e70090. doi: 10.1002/jev2.70090. J Extracell Vesicles. 2025. PMID: 40415221 Free PMC article.
-
Colletotrichum higginsianum as a Model for Understanding Host⁻Pathogen Interactions: A Review.Int J Mol Sci. 2018 Jul 23;19(7):2142. doi: 10.3390/ijms19072142. Int J Mol Sci. 2018. PMID: 30041456 Free PMC article. Review.
-
A two-way road: novel roles for fungal extracellular vesicles.Mol Microbiol. 2018 Oct;110(1):11-15. doi: 10.1111/mmi.14095. Epub 2018 Oct 4. Mol Microbiol. 2018. PMID: 30079549 Review.
Cited by
-
Extracellular Vesicles of the Plant Pathogen Botrytis cinerea.J Fungi (Basel). 2023 Apr 20;9(4):495. doi: 10.3390/jof9040495. J Fungi (Basel). 2023. PMID: 37108947 Free PMC article.
-
The Adaptation of Botrytis cinerea Extracellular Vesicles Proteome to Surrounding Conditions: Revealing New Tools for Its Infection Process.J Fungi (Basel). 2023 Aug 24;9(9):872. doi: 10.3390/jof9090872. J Fungi (Basel). 2023. PMID: 37754980 Free PMC article.
-
Extracellular Vesicles from Scedosporium apiospermum Mycelial Cells: Implication for Fungal-Host Interplays.J Fungi (Basel). 2024 Apr 9;10(4):277. doi: 10.3390/jof10040277. J Fungi (Basel). 2024. PMID: 38667948 Free PMC article.
-
Establishment of an experimental system to analyse extracellular vesicles during apoplastic fungal pathogenesis.J Extracell Biol. 2025 Feb 17;4(2):e70029. doi: 10.1002/jex2.70029. eCollection 2025 Feb. J Extracell Biol. 2025. PMID: 39963377 Free PMC article.
-
Extracellular vesicles in phytopathogenic fungi.Extracell Vesicles Circ Nucl Acids. 2023 Mar 30;4(1):90-106. doi: 10.20517/evcna.2023.04. eCollection 2023. Extracell Vesicles Circ Nucl Acids. 2023. PMID: 39698296 Free PMC article. Review.
References
-
- Aalto, M. K. , Ronne, H. , & Keranen, S. (1993). Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. Embo Journal, 12(11), 4095–4104. https://www.ncbi.nlm.nih.gov/pubmed/8223426 - PMC - PubMed
-
- Albuquerque, P. C. , Nakayasu, E. S. , Rodrigues, M. L. , Frases, S. , Casadevall, A. , Zancope‐Oliveira, R. M. , Almeida, I. C. , & Nosanchuk, J. D. (2008). Vesicular transport in Histoplasma capsulatum: An effective mechanism for trans‐cell wall transfer of proteins and lipids in ascomycetes. Cellular Microbiology, 10(8), 1695–1710. 10.1111/j.1462-5822.2008.01160.x - DOI - PMC - PubMed
-
- Almagro Armenteros, J. J. , Tsirigos, K. D. , Sonderby, C. K. , Petersen, T. N. , Winther, O. , Brunak, S. , von Heijne, G. , & Nielsen, H. (2019). SignalP 5.0 improves signal peptide predictions using deep neural networks. Nature Biotechnology, 37(4), 420–423. 10.1038/s41587-019-0036-z - DOI - PubMed

