Tetraspanins distinguish separate extracellular vesicle subpopulations in human serum and plasma - Contributions of platelet extracellular vesicles in plasma samples
- PMID: 35524458
- PMCID: PMC9077141
- DOI: 10.1002/jev2.12213
Tetraspanins distinguish separate extracellular vesicle subpopulations in human serum and plasma - Contributions of platelet extracellular vesicles in plasma samples
Abstract
Background: The ability to isolate extracellular vesicles (EVs) from blood is vital in the development of EVs as disease biomarkers. Both serum and plasma can be used, but few studies have compared these sources in terms of the type of EVs that are obtained. The aim of this study was to determine the presence of different subpopulations of EVs in plasma and serum.
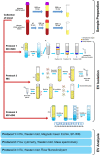
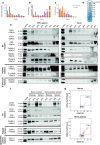
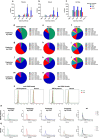
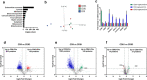
Method: Blood was collected from healthy subjects, and plasma and serum were isolated in parallel. ACD or EDTA tubes were used for the collection of plasma, while serum was obtained in clot activator tubes. EVs were isolated utilising a combination of density cushion and SEC, a combination of density cushion and gradient or by a bead antibody capturing system (anti-CD63, anti-CD9 and anti-CD81 beads). The subpopulations of EVs were analysed by NTA, Western blot, SP-IRIS, conventional and nano flow cytometry, magnetic bead ELISA and mass spectrometry. Additionally, different isolation protocols for plasma were compared to determine the contribution of residual platelets in the analysis.
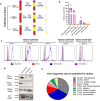
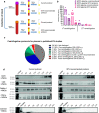
Results: This study shows that a higher number of CD9+ EVs were present in EDTA-plasma compared to ACD-plasma and to serum, and the presence of CD41a on these EVs suggests that they were released from platelets. Furthermore, only a very small number of EVs in blood were double-positive for CD63 and CD81. The CD63+ EVs were enriched in serum, while CD81+ vesicles were the rarest subpopulation in both plasma and serum. Additionally, EDTA-plasma contained more residual platelets than ACD-plasma and serum, and two centrifugation steps were crucial to reduce the number of platelets in plasma prior to EV isolation.
Conclusion: These results show that human blood contains multiple subpopulations of EVs that carry different tetraspanins. Blood sampling methods, including the use of anti-coagulants and choice of centrifugation protocols, can affect EV analyses and should always be reported in detail.
Keywords: biomarkers; exosomes; extracellular vesicles; microvesicles; plasma; serum; subpopulations.
© 2022 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
J.L. and C.L. have developed multiple EV‐associated patents for putative clinical utilization. J.L. owns equity in Codiak BioSciences Inc. and Exocure Biosciences Inc. and consults in the field of EVs through Vesiclebio AB. C.L. owns equity in Exocure Bioscience Inc.
Figures


References
-
- Bachurski, D. , Schuldner, M. , Nguyen, P. H. , Malz, A. , Reiners, K. S. , & Grenzi, P. C. (2019). Extracellular vesicle measurements with nanoparticle tracking analysis ‐ An accuracy and repeatability comparison between NanoSight NS300 and ZetaView. Journal of Extracellular Vesicles, 8(1), 1596016. - PMC - PubMed
-
- Baek, R. , Sondergaard, E. K. , Varming, K. , & Jorgensen, M. M. (2016). The impact of various preanalytical treatments on the phenotype of small extracellular vesicles in blood analyzed by protein microarray. Journal of Immunological Methods, 438, 11–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

