Targeted delivery to macrophages and dendritic cells by chemically modified mannose ligand-conjugated siRNA
- PMID: 35524566
- PMCID: PMC9122583
- DOI: 10.1093/nar/gkac308
Targeted delivery to macrophages and dendritic cells by chemically modified mannose ligand-conjugated siRNA
Abstract
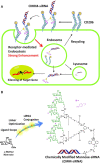
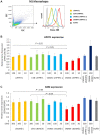
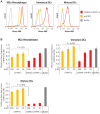
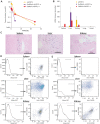
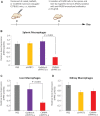
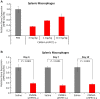
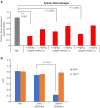
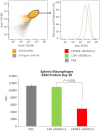
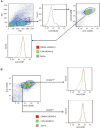
Extrahepatic delivery of small interfering RNAs (siRNAs) may have applications in the development of novel therapeutic approaches. However, reports on such approaches are limited, and the scarcity of reports concerning the systemically targeted delivery of siRNAs with effective gene silencing activity presents a challenge. We herein report for the first time the targeted delivery of CD206-targetable chemically modified mannose-siRNA (CMM-siRNA) conjugates to macrophages and dendritic cells (DCs). CMM-siRNA exhibited a strong binding ability to CD206 and selectively delivered contents to CD206-expressing macrophages and DCs. Furthermore, the conjugates demonstrated strong gene silencing ability with long-lasting effects and protein downregulation in CD206-expressing cells in vivo. These findings could broaden the use of siRNA technology, provide additional therapeutic opportunities, and establish a basis for further innovative approaches for the targeted delivery of siRNAs to not only macrophages and DCs but also other cell types.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

Similar articles
-
Enhancement of Gene Knockdown on CD22-Expressing Cells by Chemically Modified Glycan Ligand-siRNA Conjugates.ACS Chem Biol. 2022 Feb 18;17(2):292-298. doi: 10.1021/acschembio.1c00652. Epub 2022 Jan 12. ACS Chem Biol. 2022. PMID: 35020348
-
Design of Mannose-Functionalized Curdlan Nanoparticles for Macrophage-Targeted siRNA Delivery.ACS Appl Mater Interfaces. 2018 May 2;10(17):14463-14474. doi: 10.1021/acsami.8b02073. Epub 2018 Apr 19. ACS Appl Mater Interfaces. 2018. PMID: 29648784
-
Combination treatment of mannose and GalNAc conjugated small interfering RNA protects against lethal Marburg virus infection.Mol Ther. 2023 Jan 4;31(1):269-281. doi: 10.1016/j.ymthe.2022.09.009. Epub 2022 Sep 15. Mol Ther. 2023. PMID: 36114672 Free PMC article.
-
Nonviral in vivo delivery of therapeutic small interfering RNAs.Curr Opin Mol Ther. 2007 Aug;9(4):345-52. Curr Opin Mol Ther. 2007. PMID: 17694447 Review.
-
In vivo cellular delivery of siRNA.IDrugs. 2010 Jun;13(6):383-7. IDrugs. 2010. PMID: 20506060 Review.
Cited by
-
Fatty Acid Derivatization and Cyclization of the Immunomodulatory Peptide RP-182 Targeting CD206high Macrophages Improve Antitumor Activity.Mol Cancer Ther. 2024 Dec 3;23(12):1827-1841. doi: 10.1158/1535-7163.MCT-23-0790. Mol Cancer Ther. 2024. PMID: 39212669 Free PMC article.
-
Myeloid but not hepatocytic CD38 is a key driver for hepatic ischemia/reperfusion injury.Signal Transduct Target Ther. 2025 May 9;10(1):150. doi: 10.1038/s41392-025-02233-8. Signal Transduct Target Ther. 2025. PMID: 40341132 Free PMC article.
-
Functional Polarization of Liver Macrophages by Glyco Gold Nanoparticles.Adv Sci (Weinh). 2025 Apr;12(16):e2407458. doi: 10.1002/advs.202407458. Epub 2025 Feb 14. Adv Sci (Weinh). 2025. PMID: 39950558 Free PMC article.
-
Future directions for medicinal chemistry in the field of oligonucleotide therapeutics.RNA. 2023 Apr;29(4):423-433. doi: 10.1261/rna.079511.122. Epub 2023 Jan 24. RNA. 2023. PMID: 36693762 Free PMC article.
-
Double-targeted liposomes coated with matrix metallopeptidase-2-responsive polypeptide nanogel for chemotherapy and enhanced immunotherapy against cervical cancer.Mater Today Bio. 2024 Dec 17;30:101412. doi: 10.1016/j.mtbio.2024.101412. eCollection 2025 Feb. Mater Today Bio. 2024. PMID: 39811606 Free PMC article.
References
-
- Setten R.L., Rossi J.J., Han S.. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug. Discov. 2019; 18:421–446. - PubMed
-
- Nikam R.R., Gore K.R.. Journey of siRNA: clinical developments and targeted delivery. Nucleic Acid Ther. 2018; 28:209–224. - PubMed
-
- Editorial Delivering the promise of RNA therapeutics. Nat. Med. 2019; 25:1321. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

