Unlocking New Reactivities in Enzymes by Iminium Catalysis
- PMID: 35524737
- PMCID: PMC9400869
- DOI: 10.1002/anie.202203613
Unlocking New Reactivities in Enzymes by Iminium Catalysis
Abstract
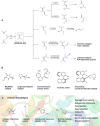
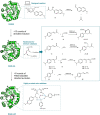
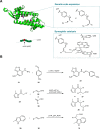
The application of biocatalysis in conquering challenging synthesis requires the constant input of new enzymes. Developing novel biocatalysts by absorbing catalysis modes from synthetic chemistry has yielded fruitful new-to-nature enzymes. Organocatalysis was originally bio-inspired and has become the third pillar of asymmetric catalysis. Transferring organocatalytic reactions back to enzyme platforms is a promising approach for biocatalyst creation. Herein, we summarize recent developments in the design of novel biocatalysts that adopt iminium catalysis, a fundamental branch in organocatalysis. By repurposing existing enzymes or constructing artificial enzymes, various biocatalysts for iminium catalysis have been created and optimized via protein engineering to promote valuable abiological transformations. Recent advances in iminium biocatalysis illustrate the power of combining chemomimetic biocatalyst design and directed evolution to generate useful new-to-nature enzymes.
Keywords: Biocatalysis; Chemomimetic Catalysis; Iminium Ions; Organocatalysis.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Designing Michaelases: exploration of novel protein scaffolds for iminium biocatalysis.Faraday Discuss. 2024 Sep 11;252(0):279-294. doi: 10.1039/d4fd00057a. Faraday Discuss. 2024. PMID: 38842386 Free PMC article.
-
Engineering the third wave of biocatalysis.Nature. 2012 May 9;485(7397):185-94. doi: 10.1038/nature11117. Nature. 2012. PMID: 22575958 Review.
-
Improving and repurposing biocatalysts via directed evolution.Curr Opin Chem Biol. 2015 Apr;25:55-64. doi: 10.1016/j.cbpa.2014.12.036. Epub 2015 Jan 8. Curr Opin Chem Biol. 2015. PMID: 25579451 Review.
-
Chemomimetic biocatalysis: exploiting the synthetic potential of cofactor-dependent enzymes to create new catalysts.J Am Chem Soc. 2015 Nov 11;137(44):13992-4006. doi: 10.1021/jacs.5b09348. Epub 2015 Nov 3. J Am Chem Soc. 2015. PMID: 26502343
-
Design and Evolution of an Enzyme for the Asymmetric Michael Addition of Cyclic Ketones to Nitroolefins by Enamine Catalysis.Angew Chem Int Ed Engl. 2024 Aug 12;63(33):e202404312. doi: 10.1002/anie.202404312. Epub 2024 Jul 9. Angew Chem Int Ed Engl. 2024. PMID: 38783596
Cited by
-
Designing Michaelases: exploration of novel protein scaffolds for iminium biocatalysis.Faraday Discuss. 2024 Sep 11;252(0):279-294. doi: 10.1039/d4fd00057a. Faraday Discuss. 2024. PMID: 38842386 Free PMC article.
-
Enhancing the Peroxygenase Activity of a Cofactor-Independent Peroxyzyme by Directed Evolution Enabling Gram-Scale Epoxide Synthesis.Chemistry. 2022 Oct 21;28(59):e202201651. doi: 10.1002/chem.202201651. Epub 2022 Aug 26. Chemistry. 2022. PMID: 35861144 Free PMC article.
-
Chemical Switching: A Concept Inspired by Strategies from Biocatalysis and Organocatalysis.Chembiochem. 2025 Jun 3;26(11):e202500220. doi: 10.1002/cbic.202500220. Epub 2025 May 26. Chembiochem. 2025. PMID: 40417833 Free PMC article. Review.
-
Stereospecific radical coupling with a non-natural photodecarboxylase.Nature. 2024 Oct;634(8035):848-854. doi: 10.1038/s41586-024-08004-9. Epub 2024 Sep 10. Nature. 2024. PMID: 39255850
-
Transitioning enzyme catalysis towards photocatalysis.Philos Trans A Math Phys Eng Sci. 2025 May 8;383(2296):20230380. doi: 10.1098/rsta.2023.0380. Epub 2025 May 8. Philos Trans A Math Phys Eng Sci. 2025. PMID: 40336288 Free PMC article. Review.
References
-
- Bell E. L., Finnigan W., France S. P., Green A. P., Hayes M. A., Hepworth L. J., Lovelock S. L., Niikura H., Osuna S., Romero E., Ryan K. S., Turner N. J., Flitsch S. L., Nat. Rev. Methods Primers 2021, 1, 46.
-
- Bornscheuer U. T., Huisman G. W., Kazlauskas R. J., Lutz S., Moore J. C., Robins K., Nature 2012, 485, 185–194. - PubMed
-
- Turner N. J., O'Reilly E., Nat. Chem. Biol. 2013, 9, 285–288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

