Harnessing retinal phagocytes to combat pathological neovascularization in ischemic retinopathies?
- PMID: 35524802
- PMCID: PMC9117346
- DOI: 10.1007/s00424-022-02695-7
Harnessing retinal phagocytes to combat pathological neovascularization in ischemic retinopathies?
Abstract
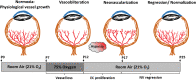
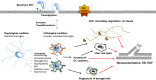
Ischemic retinopathies (IR) are vision-threatening diseases that affect a substantial amount of people across all age groups worldwide. The current treatment options of photocoagulation and anti-VEGF therapy have side effects and are occasionally unable to prevent disease progression. It is therefore worthwhile to consider other molecular targets for the development of novel treatment strategies that could be safer and more efficient. During the manifestation of IR, the retina, normally an immune privileged tissue, encounters enhanced levels of cellular stress and inflammation that attract mononuclear phagocytes (MPs) from the blood stream and activate resident MPs (microglia). Activated MPs have a multitude of effects within the retinal tissue and have the potential to both counter and exacerbate the harmful tissue microenvironment. The present review discusses the current knowledge about the role of inflammation and activated retinal MPs in the major IRs: retinopathy of prematurity and diabetic retinopathy. We focus particularly on MPs and their secreted factors and cell-cell-based interactions between MPs and endothelial cells. We conclude that activated MPs play a major role in the manifestation and progression of IRs and could therefore become a promising new target for novel pharmacological intervention strategies in these diseases.
Keywords: Inflammation; Macrophages; Microglia; Mononuclear phagocytes; Pathological angiogenesis; Retinopathy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
The authors declare no competing interests.
Figures


References
-
- Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227–1239. - PubMed
-
- Arroba AI, et al. Modulation of microglia polarization dynamics during diabetic retinopathy in db/db mice. Biochim Biophys Acta. 2016;1862(9):1663–1674. - PubMed
-
- Barile GR, et al. The RAGE axis in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005;46(8):2916–2924. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

