Switching to Preservative-Free Tafluprost/Timolol Fixed-Dose Combination in the Treatment of Open-Angle Glaucoma or Ocular Hypertension: Subanalysis of Data from the VISIONARY Study According to Baseline Monotherapy Treatment
- PMID: 35524840
- PMCID: PMC9309126
- DOI: 10.1007/s12325-022-02166-6
Switching to Preservative-Free Tafluprost/Timolol Fixed-Dose Combination in the Treatment of Open-Angle Glaucoma or Ocular Hypertension: Subanalysis of Data from the VISIONARY Study According to Baseline Monotherapy Treatment
Erratum in
-
Correction to: Switching to Preservative-Free Tafluprost/Timolol Fixed-Dose Combination in the Treatment of Open-Angle Glaucoma or Ocular Hypertension: Subanalysis of Data from the VISIONARY Study According to Baseline Monotherapy Treatment.Adv Ther. 2022 Aug;39(8):3522-3523. doi: 10.1007/s12325-022-02210-5. Adv Ther. 2022. PMID: 35731341 Free PMC article. No abstract available.
Abstract
Introduction: The VISIONARY study demonstrated statistically significant intraocular pressure (IOP) reductions with the preservative-free fixed-dose combination of tafluprost 0.0015% and timolol 0.5% (PF tafluprost/timolol FC) in open-angle glaucoma (OAG) or ocular hypertension (OHT) patients, sub-optimally controlled with topical prostaglandin analogue (PGA) or beta-blocker monotherapy. Current subanalyses have examined these data according to the baseline monotherapy.
Methods: A European, prospective, observational study included adults (aged ≥ 18 years) with OAG or OHT, who were switched to the PF tafluprost/timolol FC from PGA or beta-blocker monotherapy. Treatment outcomes were reported according to prior monotherapy subgroup: beta-blocker, preserved latanoprost, PF-latanoprost, bimatoprost, tafluprost, and travoprost. Endpoints included the mean change from baseline regarding IOP, conjunctival hyperemia, and corneal fluorescein staining (CFS) at Week 4 and Week 12, and at Month 6.
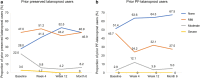
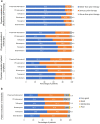
Results: The subanalysis included 577 patients. All prior monotherapy subgroups demonstrated statistically significant IOP reductions from baseline at Week 4, that were maintained through Month 6 (p < 0.001). Mean (SD) IOP change at Month 6 was 6.6 (4.16), 6.3 (4.39), 5.6 (3.67), 4.9 (2.97), 4.6 (4.39), and 4.7 (3.64) mmHg for prior beta-blocker, preserved latanoprost, PF-latanoprost, tafluprost, bimatoprost, and travoprost subgroups, respectively. The largest IOP change was observed in the preserved latanoprost subgroup for each of the ≥ 20%, ≥ 25%, ≥ 30%, and ≥ 35% IOP reduction categories at Month 6, demonstrating respective reductions of 8.06, 9.20, 10.64, and 11.55 mmHg. CFS was significantly reduced at Month 6 in the prior bimatoprost subgroup (p = 0.0013). Conjunctival hyperemia severity was significantly reduced at each study visit for prior preserved latanoprost users (p < 0.001).
Conclusion: PF tafluprost/timolol FC therapy provided statistically and clinically significant IOP reductions from Week 4 over the total 6-month period, in patients with OAG/OHT, regardless of the type of prior PGA or beta-blocker monotherapy used. Conjunctival hyperemia severity and CFS decreased significantly in prior bimatoprost and preserved latanoprost users, respectively.
Clinical study number: European Union electronic Register of Post-Authorization Studies (EU PAS) register number: EUPAS22204.
Keywords: Beta-blocker monotherapy; Ocular hypertension; Open-angle glaucoma; Preservative-free topical medication; Prostaglandin analogue monotherapy; Real-world evidence; Tafluprost/timolol fixed-dose combination; VISIONARY study.
© 2022. The Author(s).
Figures
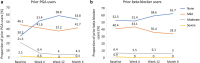
References
-
- European Glaucoma Society. Terminology and guidelines for glaucoma 5th Edition. 2020. Available at https://www.eugs.org/eng/guidelines.asp. Accessed June 2021. - PubMed
-
- International Council of Ophthalmology. Guidelines for glaucoma care. 2016. http://www.icoph.org/downloads/ICOGlaucomaGuidelines.pdf. Accessed October 12, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

