Value of [68Ga]Ga-somatostatin receptor PET/CT in the grading of pulmonary neuroendocrine (carcinoid) tumours and the detection of disseminated disease: single-centre pathology-based analysis and review of the literature
- PMID: 35524900
- PMCID: PMC9079198
- DOI: 10.1186/s13550-022-00900-3
Value of [68Ga]Ga-somatostatin receptor PET/CT in the grading of pulmonary neuroendocrine (carcinoid) tumours and the detection of disseminated disease: single-centre pathology-based analysis and review of the literature
Abstract
Background: Although most guidelines suggest performing a positron emission tomography/computed tomography (PET/CT) with somatostatin receptor (SSTR) ligands for staging of pulmonary carcinoid tumours (PC), only a limited number of studies have evaluated the role of this imaging tool in this specific patient population. The preoperative differentiation between typical carcinoid (TC) and atypical carcinoid (AC) and the extent of dissemination (N/M status) are crucial factors for treatment allocation and prognosis of these patients. Therefore, we performed a pathology-based retrospective analysis of the value of SSTR PET/CT in tumour grading and detection of nodal and metastatic involvement of PC and compared this with the previous literature and with [18F]FDG PET/CT in a subgroup of patients.
Methods: SSTR PET/CT scans performed between January 2007 and May 2020 in the context of PC were included. If available, [18F]FDG PET/CT images were also evaluated. The maximum standardized uptake (SUVmax) values of the primary tumour, of the pathologically examined hilar and mediastinal lymph node stations, as well as of the distant metastases, were recorded. Tumoural SUVmax values were related to the tumour type (TC versus AC) for both SSTR and [18F]FDG PET/CT in diagnosing and differentiating both tumour types. Nodal SUVmax values were compared to the pathological status (N+ versus N-) to evaluate the diagnostic accuracy of SSTR PET/CT in detecting lymph node involvement. Finally, a mixed model analysis of all pathologically proven distant metastatic lesions was performed.
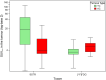
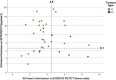
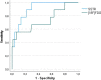
Results: A total of 86 SSTR PET/CT scans performed in 86 patients with PC were retrospectively analysed. [18F]FDG PET/CT was available in 46 patients. Analysis of the SUVmax values in the primary tumour showed significantly higher SSTR uptake in TC compared with AC (median SUVmax 18.4 vs 3.8; p = 0.003) and significantly higher [18F]FDG uptake in AC compared to TC (median SUVmax 5.4 vs 3.5; p = 0.038). Receiver operating characteristic (ROC) curve analysis resulted in an area under the curve (AUC) of 0.78 for the detection of TC on SSTR PET/CT and of 0.73 for the detection of AC on [18F]FDG PET/CT. A total of 267 pathologically evaluated hilar and mediastinal lymph node stations were analysed. ROC analysis of paired SSTR/[18F]FDG SUVmax values for the detection of metastasis of TC in 83 lymph node stations revealed an AUC of 0.91 for SSTR PET/CT and of 0.74 for [18F]FDG PET/CT (difference 0.17; 95% confidence interval - 0.03 to 0.38; p = 0.10). In a sub-cohort of 10 patients with 12 distant lesions that were pathologically examined due to a suspicious aspect on SSTR PET/CT, a positive predictive value (PPV) of 100% was observed.
Conclusion: Our findings confirm the higher SSTR ligand uptake in TC compared to AC and vice versa for [18F]FDG uptake. More importantly, we found a good diagnostic performance of SSTR PET/CT for the detection of hilar and mediastinal lymph node metastases of TC. Finally, a PPV of 100% for SSTR PET/CT was found in a small sub-cohort of patients with pathologically investigated distant metastatic lesions. Taken together, SSTR PET/CT has a very high diagnostic value in the TNM assessment of pulmonary carcinoids, particularly in TC, which underscores its position in European guidelines.
Keywords: Atypical carcinoid; Bronchial carcinoid; Neuroendocrine tumour; PET; Pulmonary carcinoid; Somatostatin receptor; Typical carcinoid; [18F]FDG; [68Ga]Ga-DOTATATE; [68Ga]Ga-DOTATOC.
© 2022. The Author(s).
Conflict of interest statement
CMD has worked as a consultant for Terumo, SIRTex and PSI CRO and as speaker for IPSEN; all funds were received by his institution. The other authors declare that they have no competing interests.
Figures


Similar articles
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
Comparison of (18F)FDG PET/CT and (68Ga)DOTATATE PET/CT imaging methods in terms of detection of histological subtype and related SUVmax values in patients with pulmonary carcinoid tumors.Nucl Med Commun. 2019 May;40(5):517-524. doi: 10.1097/MNM.0000000000000985. Nucl Med Commun. 2019. PMID: 30694875
-
The utility of 18F-FDG and 68Ga-DOTA-Peptide PET/CT in the evaluation of primary pulmonary carcinoid: A systematic review and meta-analysis.Medicine (Baltimore). 2019 Mar;98(10):e14769. doi: 10.1097/MD.0000000000014769. Medicine (Baltimore). 2019. PMID: 30855482 Free PMC article.
-
Somatostatin receptor-targeted theranostics in patients with estrogen receptor-positive metastatic breast cancer-a prospective exploratory study.Breast Cancer Res Treat. 2025 Jun;211(2):363-373. doi: 10.1007/s10549-025-07651-4. Epub 2025 Feb 26. Breast Cancer Res Treat. 2025. PMID: 40000538
-
Comparison of gallium-68 somatostatin receptor and 18F-fluorodeoxyglucose positron emission tomography in the diagnosis of neuroendocrine tumours: A systematic review and meta-analysis.Hell J Nucl Med. 2020 May-Aug;23(2):188-200. doi: 10.1967/s002449912108. Epub 2020 Jul 27. Hell J Nucl Med. 2020. PMID: 32716410
Cited by
-
Diagnostic accuracy of SSR-PET/CT compared to histopathology in the identification of liver metastases from well-differentiated neuroendocrine tumors.Cancer Imaging. 2023 Sep 28;23(1):92. doi: 10.1186/s40644-023-00614-2. Cancer Imaging. 2023. PMID: 37770958 Free PMC article.
-
Positron emission tomography in the diagnosis and management of primary pediatric lung tumors.Pediatr Radiol. 2024 May;54(5):671-683. doi: 10.1007/s00247-023-05847-8. Epub 2024 Jan 17. Pediatr Radiol. 2024. PMID: 38231400 Review.
-
Occupational radiation exposure assessment during the management of [68Ga]Ga-DOTA-TOC.EJNMMI Phys. 2022 Oct 29;9(1):75. doi: 10.1186/s40658-022-00505-8. EJNMMI Phys. 2022. PMID: 36309605 Free PMC article.
-
A Systematic Review on Combined [18F]FDG and 68Ga-SSA PET/CT in Pulmonary Carcinoid.J Clin Med. 2023 May 28;12(11):3719. doi: 10.3390/jcm12113719. J Clin Med. 2023. PMID: 37297914 Free PMC article. Review.
-
Updates on Pulmonary Neuroendocrine Carcinoids: Progress and Perspectives.J Clin Med. 2025 Aug 13;14(16):5733. doi: 10.3390/jcm14165733. J Clin Med. 2025. PMID: 40869558 Free PMC article. Review.
References
-
- Caplin ME, Baudin E, Ferolla P, Filosso P, Garcia-Yuste M, Lim E, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26:1604–1620. doi: 10.1093/annonc/mdv041. - DOI - PubMed
-
- Singh S, Bergsland EK, Card CM, Hope TA, Kunz PL, Laidley DT, et al. Commonwealth Neuroendocrine Tumour Research Collaboration and the North American Neuroendocrine Tumor Society Guidelines for the Diagnosis and Management of Patients With Lung Neuroendocrine Tumors: an International Collaborative Endorsement and Update of. J Thorac Oncol. 2020;15:1577–1598. doi: 10.1016/j.jtho.2020.06.021. - DOI - PubMed
LinkOut - more resources
Full Text Sources

