ApoA-I Infusion Therapies Following Acute Coronary Syndrome: Past, Present, and Future
- PMID: 35524914
- PMCID: PMC9236992
- DOI: 10.1007/s11883-022-01025-7
ApoA-I Infusion Therapies Following Acute Coronary Syndrome: Past, Present, and Future
Abstract
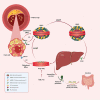
Purpose of review: The elevated adverse cardiovascular event rate among patients with low high-density lipoprotein cholesterol (HDL-C) formed the basis for the hypothesis that elevating HDL-C would reduce those events. Attempts to raise endogenous HDL-C levels, however, have consistently failed to show improvements in cardiovascular outcomes. However, steady-state HDL-C concentration does not reflect the function of this complex family of particles. Indeed, HDL functions correlate only weakly with serum HDL-C concentration. Thus, the field has pivoted from simply raising the quantity of HDL-C to a focus on improving the putative anti-atherosclerotic functions of HDL particles. Such functions include the ability of HDL to promote the efflux of cholesterol from cholesterol-laden macrophages. Apolipoprotein A-I (apoA-I), the signature apoprotein of HDL, may facilitate the removal of cholesterol from atherosclerotic plaque, reduce the lesional lipid content and might thus stabilize vulnerable plaques, thereby reducing the risk of cardiac events. Infusion of preparations of apoA-I may improve cholesterol efflux capacity (CEC). This review summarizes the development of apoA-I therapies, compares their structural and functional properties and discusses the findings of previous studies including their limitations, and how CSL112, currently being tested in a phase III trial, may overcome these challenges.
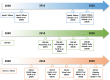
Recent findings: Three major ApoA-I-based approaches (MDCO-216, CER-001, and CSL111/CSL112) have aimed to enhance reverse cholesterol transport. These three therapies differ considerably in both lipid and protein composition. MDCO-216 contains recombinant ApoA-I Milano, CER-001 contains recombinant wild-type human ApoA-I, and CSL111/CSL112 contains native ApoA-I isolated from human plasma. Two of the three agents studied to date (apoA-1 Milano and CER-001) have undergone evaluation by intravascular ultrasound imaging, a technique that gauges lesion volume well but does not assess other important variables that may relate to clinical outcomes. ApoA-1 Milano and CER-001 reduce lecithin-cholesterol acyltransferase (LCAT) activity, potentially impairing the function of HDL in reverse cholesterol transport. Furthermore, apoA-I Milano can compete with and alter the function of the recipient's endogenous apoA-I. In contrast to these agents, CSL112, a particle formulated using human plasma apoA-I and phosphatidylcholine, increases LCAT activity and does not lead to the malfunction of endogenous apoA-I. CSL112 robustly increases cholesterol efflux, promotes reverse cholesterol transport, and now is being tested in a phase III clinical trial. Phase II-b studies of MDCO-216 and CER-001 failed to produce a significant reduction in coronary plaque volume as assessed by IVUS. However, the investigation to determine whether the direct infusion of a reconstituted apoA-I reduces post-myocardial infarction coronary events is being tested using CSL112, which is dosed at a higher level than MDCO-216 and CER-001 and has more favorable pharmacodynamics.
Keywords: Acute coronary syndrome; ApoA-I infusion therapies; Cholesterol efflux capacity.
© 2022. The Author(s).
Conflict of interest statement
Dr. Gibson receives consultant fees from Portola Pharmaceuticals and reports grants from Angel Medical Corporation and CSL Behring; grants and other support from Bayer Corporation; grants and personal fees from Janssen, Johnson & Johnson, and Portola Pharmaceuticals; and personal fees from The Medicines Company, Boston Clinical Research Institute, Cardiovascular Research Foundation, Eli Lilly, Gilead Sciences Inc., Novo Nordisk, Pfizer, Web MD, UpToDate in Cardiovascular Medicine, Amarin Pharma, Amgen, Arena Pharmaceuticals, Bayer Corporation, Boehringer Ingelheim, Chiesi, Merck & Co., PharmaMar, Sanofi, Somahlution, St. Francis Hospital, and Verreseon Corporation. Dr. Libby is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, Medimmune, Merck, Norvo Nordisk, Novartis, Pfizer, and Sanofi-Regeneron. Dr. Libby is a member of the scientific advisory board for Amgen, Caristo, Cartesian, CSL Behring, DalCor Pharmaceuticals, Dewpoint, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, PlaqueTec, and XBiotech, Inc. Dr. Libby’s laboratory has received research funding in the last 2 years from Novartis. Dr. Libby is on the Board of Directors of XBiotech, Inc. Dr. Peter Libby has a financial interest in TenSixteen Bio, a company targeting somatic mosaicism and clonal hematopoiesis of indeterminate potential (CHIP) to discover and develop novel therapeutics to treat age-related diseases. Dr. Libby has a financial interest in Xbiotech, a company developing therapeutic human antibodies. Dr. Libby’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict-of-interest policies. Dr. Libby receives funding support from the National Heart, Lung, and Blood Institute (1R01HL134892), the American Heart Association (18CSA34080399), the RRM Charitable Fund, and the Simard Fund. Dr. Libby receives funding support from the National Heart, Lung, and Blood Institute (1R01HL134892), the American Heart Association (18CSA34080399), the RRM Charitable Fund, and the Simard Fund. Dr. Wright, Dr. Kingwell, Dr. Tricoci, Dr. Shaunik, Dr. Berman, and Dr. Duffy are employed by CSL Behring. All remaining authors declare no conflict of interest.
Figures
Similar articles
-
CSL112 (Apolipoprotein A-I [Human]) Enhances Cholesterol Efflux Similarly in Healthy Individuals and Stable Atherosclerotic Disease Patients.Arterioscler Thromb Vasc Biol. 2018 Apr;38(4):953-963. doi: 10.1161/ATVBAHA.118.310538. Epub 2018 Feb 8. Arterioscler Thromb Vasc Biol. 2018. PMID: 29437574 Free PMC article. Clinical Trial.
-
A single infusion of MDCO-216 (ApoA-1 Milano/POPC) increases ABCA1-mediated cholesterol efflux and pre-beta 1 HDL in healthy volunteers and patients with stable coronary artery disease.Eur Heart J Cardiovasc Pharmacother. 2016 Jan;2(1):23-9. doi: 10.1093/ehjcvp/pvv041. Epub 2015 Dec 11. Eur Heart J Cardiovasc Pharmacother. 2016. PMID: 27418968 Free PMC article. Clinical Trial.
-
CSL112 (Apolipoprotein A-I [Human]) Strongly Enhances Plasma Apoa-I and Cholesterol Efflux Capacity in Post-Acute Myocardial Infarction Patients: A PK/PD Substudy of the AEGIS-I Trial.J Cardiovasc Pharmacol Ther. 2022 Jan-Dec;27:10742484221121507. doi: 10.1177/10742484221121507. J Cardiovasc Pharmacol Ther. 2022. PMID: 36282079 Clinical Trial.
-
CSL112, a reconstituted, infusible, plasma-derived apolipoprotein A-I: safety and tolerability profiles and implications for management in patients with myocardial infarction.Expert Opin Investig Drugs. 2018 Dec;27(12):997-1005. doi: 10.1080/13543784.2018.1543399. Epub 2018 Nov 3. Expert Opin Investig Drugs. 2018. PMID: 30376729 Review.
-
Biological basis and proposed mechanism of action of CSL112 (apolipoprotein A-I [human]) for prevention of major adverse cardiovascular events in patients with myocardial infarction.Eur Heart J Cardiovasc Pharmacother. 2023 Jun 2;9(4):387-398. doi: 10.1093/ehjcvp/pvad014. Eur Heart J Cardiovasc Pharmacother. 2023. PMID: 36787889 Free PMC article. Review.
Cited by
-
Impact of a Plant Sterol Food Supplement on Eryptotic and Associated Cardiometabolic Parameters: A Randomized Placebo-Controlled Trial in Statin-Treated Patients.Foods. 2024 Dec 19;13(24):4108. doi: 10.3390/foods13244108. Foods. 2024. PMID: 39767050 Free PMC article.
-
High-Density Lipoproteins at the Interface between the NLRP3 Inflammasome and Myocardial Infarction.Int J Mol Sci. 2024 Jan 20;25(2):1290. doi: 10.3390/ijms25021290. Int J Mol Sci. 2024. PMID: 38279290 Free PMC article. Review.
-
The potential role and mechanism of circRNAs in foam cell formation.Noncoding RNA Res. 2023 Mar 21;8(3):315-325. doi: 10.1016/j.ncrna.2023.03.005. eCollection 2023 Sep. Noncoding RNA Res. 2023. PMID: 37032721 Free PMC article. Review.
-
High-density lipoproteins, Part 1. Epidemiology, antiatherogenic effects, and therapies designed to increase their serum levels.Am J Prev Cardiol. 2025 Jul 21;23:101068. doi: 10.1016/j.ajpc.2025.101068. eCollection 2025 Sep. Am J Prev Cardiol. 2025. PMID: 40787436 Free PMC article. Review.
-
Emerging Therapeutic Targets for Acute Coronary Syndromes: Novel Advancements and Future Directions.Biomedicines. 2024 Jul 26;12(8):1670. doi: 10.3390/biomedicines12081670. Biomedicines. 2024. PMID: 39200135 Free PMC article. Review.
References
-
- Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46:1225–1228. - PubMed
-
- Gofman JW, Young W, Tandy R. Ischemic heart disease, atherosclerosis, and longevity. Circulation. 1966;34:679–697. - PubMed
-
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study Am J Med. 1977;62:707–714. - PubMed
-
- Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies Circulation. 1989;79:8–15. - PubMed
-
- Barter P, Gotto AM, LaRosa JC, et al. Treating to New Targets. Investigators HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–1310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

