Human UFSP1 translated from an upstream near-cognate initiation codon functions as an active UFM1-specific protease
- PMID: 35525273
- PMCID: PMC9168615
- DOI: 10.1016/j.jbc.2022.102016
Human UFSP1 translated from an upstream near-cognate initiation codon functions as an active UFM1-specific protease
Abstract
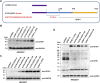
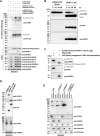
Ubiquitin-fold modifier 1 (UFM1) is a recently identified ubiquitin-like posttranslational modification with important biological functions. However, the regulatory mechanisms governing UFM1 modification of target proteins (UFMylation) and the cellular processes controlled by UFMylation remain largely unknown. It has been previously shown that a UFM1-specific protease (UFSP2) mediates the maturation of the UFM1 precursor and drives the de-UFMylation reaction. Furthermore, it has long been thought that UFSP1, an ortholog of UFSP2, is inactive in many organisms, including human, because it lacks an apparent protease domain when translated from the canonical start codon (445AUG). Here, we demonstrate using the combination of site-directed mutagenesis, CRISPR/Cas9-mediated genome editing, and mass spectrometry approaches that translation of human UFSP1 initiates from an upstream near-cognate codon, 217CUG, via eukaryotic translation initiation factor eIF2A-mediated translational initiation rather than from the annotated 445AUG, revealing the presence of a catalytic protease domain containing a Cys active site. Moreover, we show that both UFSP1 and UFSP2 mediate maturation of UFM1 and de-UFMylation of target proteins. This study demonstrates that human UFSP1 functions as an active UFM1-specific protease, thus contributing to our understanding of the UFMylation/de-UFMylation process.
Keywords: UFM1-specific protease; UFMylation; UFSP1; UFSP2; eIF2A-mediated translational initiation.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

