Fibrosis Stage-specific Incidence of Hepatocellular Cancer After Hepatitis C Cure With Direct-acting Antivirals: A Systematic Review and Meta-analysis
- PMID: 35525392
- PMCID: PMC9636072
- DOI: 10.1016/j.cgh.2022.04.013
Fibrosis Stage-specific Incidence of Hepatocellular Cancer After Hepatitis C Cure With Direct-acting Antivirals: A Systematic Review and Meta-analysis
Abstract
Background & aims: Hepatitis C virus (HCV) eradication with direct-acting antivirals reduces hepatocellular carcinoma (HCC) risk. Pooled HCC incidence rates by cirrhosis status and fibrosis stage have not been estimated using meta-analysis.
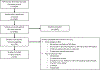
Methods: We searched PubMed, Web of Science, Embase, and Cochrane Library from January 1, 2014 to December 31, 2020 to identify studies assessing HCC incidence or outcomes by cirrhosis status, in adults with HCV who achieved sustained virologic response (SVR) after direct-acting antivirals. Pooled estimates were obtained using random-effects modeling. Subgroup, sensitivity, and meta-regression analyses were performed to evaluate heterogeneity.
Results: We included 31 studies involving 27,711 patients with cirrhosis (mean follow-up, 2.1 years) and 11 studies involving 32,123 patients without cirrhosis (mean follow-up, 2.6 years). HCC incidence was 2.99/100 person-years (95% confidence interval [CI], 2.52-3.54; I2 = 75%) in patients with cirrhosis, 0.47/100 person-years (95% CI, 0.32-0.70, I2 = 71%) in patients without cirrhosis, and 0.63/100 person-years (95% CI: 0.34-1.20, I2 = 0%) in stage 3 (F3) fibrosis. Among patients with cirrhosis, HCC incidence was highest in studies with <1 year of follow-up (6.17/100 person-years [95% CI, 3.73-10.19]) and progressively lower in studies with longer follow-up (1-2 years: 2.75/100 person-years [95% CI, 2.48-3.06]; 2-3 years: 2.90/100 person-years [95% CI, 1.90-4.44]; ≥3 years: 1.83/100 person-years [95% CI, 0.88-3.80]).
Conclusion: Pooled HCC incidence after SVR in patients with cirrhosis was very high (2.99/100 person-years) but may be declining as longer time accrues after SVR. In patients without cirrhosis, including F3 fibrosis, HCC incidence was lower than thresholds associated with cost-effective HCC screening. In patients with F3 fibrosis, the lack of between-study heterogeneity provides strong evidence that HCC screening may not be warranted.
Keywords: Cirrhosis; Fibrosis; Incidence; Liver Neoplasms.
Copyright © 2023 AGA Institute. All rights reserved.
Conflict of interest statement
Figures



Similar articles
-
Risk factors and clinical outcomes in patients with HCV eradication by direct-acting antivirals: a systematic review and meta-analysis.Infect Dis (Lond). 2025 Jul;57(7):597-627. doi: 10.1080/23744235.2025.2493370. Epub 2025 May 7. Infect Dis (Lond). 2025. PMID: 40333300 Review.
-
Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression.J Hepatol. 2017 Dec;67(6):1204-1212. doi: 10.1016/j.jhep.2017.07.025. Epub 2017 Aug 9. J Hepatol. 2017. PMID: 28802876
-
Long-Term Liver Morbidity and Mortality After Hepatitis C Virus Elimination by Direct-Acting Antivirals.J Gastroenterol Hepatol. 2025 Apr;40(4):971-978. doi: 10.1111/jgh.16892. Epub 2025 Feb 2. J Gastroenterol Hepatol. 2025. PMID: 39895100
-
NIH Consensus Statement on Management of Hepatitis C: 2002.NIH Consens State Sci Statements. 2002 Jun 10-12;19(3):1-46. NIH Consens State Sci Statements. 2002. PMID: 14768714
-
Incidence of Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-analysis, and Meta-regression.Clin Gastroenterol Hepatol. 2022 Feb;20(2):283-292.e10. doi: 10.1016/j.cgh.2021.05.002. Epub 2021 May 28. Clin Gastroenterol Hepatol. 2022. PMID: 33965578
Cited by
-
Hepatocellular carcinoma surveillance - utilization, barriers and the impact of changing aetiology.Nat Rev Gastroenterol Hepatol. 2023 Dec;20(12):797-809. doi: 10.1038/s41575-023-00818-8. Epub 2023 Aug 3. Nat Rev Gastroenterol Hepatol. 2023. PMID: 37537332 Review.
-
Differentiating Liver Metastases from Primary Liver Cancer: A Retrospective Study of Imaging and Pathological Features in Patients with Histopathological Confirmation.Biomedicines. 2025 Jan 11;13(1):164. doi: 10.3390/biomedicines13010164. Biomedicines. 2025. PMID: 39857748 Free PMC article.
-
Noninvasive Diagnosis of Hepatic Fibrosis in Hemodialysis Patients with Hepatitis C Virus Infection.Diagnostics (Basel). 2022 Sep 21;12(10):2282. doi: 10.3390/diagnostics12102282. Diagnostics (Basel). 2022. PMID: 36291971 Free PMC article. Review.
-
Beyond the Cure: Navigating Hepatocellular Risk and Surveillance after Hepatitis C Eradication in the Direct-acting Antiviral Era.J Clin Transl Hepatol. 2025 May 28;13(5):418-424. doi: 10.14218/JCTH.2024.00499. Epub 2025 Feb 8. J Clin Transl Hepatol. 2025. PMID: 40385945 Free PMC article. Review.
-
Risk stratification and early detection biomarkers for precision HCC screening.Hepatology. 2023 Jul 1;78(1):319-362. doi: 10.1002/hep.32779. Epub 2022 Oct 11. Hepatology. 2023. PMID: 36082510 Free PMC article. Review.
References
-
- Global Hepatitis Report World Health Organization, 2017.
-
- Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. World Health Organization, 2021.
-
- Kanwal F, Kramer J, Asch SM, et al. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2017;153(4):996–1005.e1. - PubMed
-
- Calvaruso V, Cabibbo G, Cacciola I, et al. Incidence of Hepatocellular Carcinoma in Patients With HCV-Associated Cirrhosis Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2018;155(2):411–21 e4. Epub 2018/04/16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

