Human papillomavirus seroprevalence and seroconversion following baseline detection of nine human papillomavirus types in young women
- PMID: 35525430
- PMCID: PMC9172167
- DOI: 10.1016/j.tvr.2022.200236
Human papillomavirus seroprevalence and seroconversion following baseline detection of nine human papillomavirus types in young women
Abstract
Background: Estimates of the humoral immune response to incident human papillomavirus (HPV) infections are limited.
Methods: In this post hoc analysis of 3875 women aged 16-23 years from a 4-valent HPV vaccine trial (NCT00092482), HPV seroprevalence on day 1 was measured with a 9-valent HPV (HPV 6/11/16/18/31/33/45/52/58) competitive Luminex immunoassay and compared with cervical/external genital HPV detection by polymerase chain reaction. In the control group, among women who were HPV DNA‒negative on day 1, seroconversion following initial HPV detection was estimated using Kaplan-Meier methods.
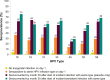
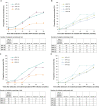
Results: Type-specific HPV seropositivity among women with no day 1 cervical/external genital HPV detection was 0.6%-3.6%. Women with any 9-valent HPV (9vHPV) cervical/external genital detection (796/3875; 20.5%) had concordant seropositivity ranging from 13.4% (HPV 45) to 38.5% (HPV 6). Among women in the control group who were negative for all HPV types on day 1, seroconversion by month 30 after initial detection ranged from 29% (HPV 45) to 75% (HPV 16).
Conclusions: Humoral immune response to HPV is variable and dynamic, depending on type-specific exposure. This longitudinal analysis provides insight into the relationship between incident infection and seropositivity.
Clinicaltrials: gov; NCT00092482 https://clinicaltrials.gov/ct2/show/NCT00092482.
Keywords: HPV infection; HPV serology; HPV vaccines; Human papillomavirus; Seroconversion; Seroprevalence.
Copyright © 2022 Merck Sharp & Dohme Corp, The Author(s). Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Vaccarella S., Franceschi S., Clifford G.M., Touze A., Hsu C.C., de Sanjose S., et al. Seroprevalence of antibodies against human papillomavirus (HPV) types 16 and 18 in four continents: the International Agency for Research on Cancer HPV prevalence Surveys. Cancer Epidemiol. Biomarkers Prev. 2010;19:2379–2388. - PubMed
-
- Carter J.J., Koutsky L.A., Hughes J.P., Lee S.K., Kuypers J., Kiviat N., et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J. Infect. Dis. 2000;181:1911–1919. - PubMed
-
- Ho G.Y.F., Studentsov Y.Y., Bierman R., Burk R.D. Natural history of human papillomavirus type 16 virus-like particle antibodies in young women. Cancer Epidemiol. Biomarkers Prev. 2004;13:110–116. - PubMed

