Effects of spike protein and toxin-like peptides found in COVID-19 patients on human 3D neuronal/glial model undergoing differentiation: Possible implications for SARS-CoV-2 impact on brain development
- PMID: 35525527
- PMCID: PMC9068247
- DOI: 10.1016/j.reprotox.2022.04.011
Effects of spike protein and toxin-like peptides found in COVID-19 patients on human 3D neuronal/glial model undergoing differentiation: Possible implications for SARS-CoV-2 impact on brain development
Abstract
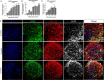
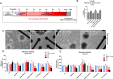
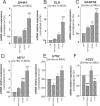
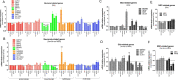
The possible neurodevelopmental consequences of SARS-CoV-2 infection are presently unknown. In utero exposure to SARS-CoV-2 has been hypothesized to affect the developing brain, possibly disrupting neurodevelopment of children. Spike protein interactors, such as ACE2, have been found expressed in the fetal brain, and could play a role in potential SARS-CoV-2 fetal brain pathogenesis. Apart from the possible direct involvement of SARS-CoV-2 or its specific viral components in the occurrence of neurological and neurodevelopmental manifestations, we recently reported the presence of toxin-like peptides in plasma, urine and fecal samples specifically from COVID-19 patients. In this study, we investigated the possible neurotoxic effects elicited upon 72-hour exposure to human relevant levels of recombinant spike protein, toxin-like peptides found in COVID-19 patients, as well as a combination of both in 3D human iPSC-derived neural stem cells differentiated for either 2 weeks (short-term) or 8 weeks (long-term, 2 weeks in suspension + 6 weeks on MEA) towards neurons/glia. Whole transcriptome and qPCR analysis revealed that spike protein and toxin-like peptides at non-cytotoxic concentrations differentially perturb the expression of SPHK1, ELN, GASK1B, HEY1, UTS2, ACE2 and some neuronal-, glia- and NSC-related genes critical during brain development. Additionally, exposure to spike protein caused a decrease of spontaneous electrical activity after two days in long-term differentiated cultures. The perturbations of these neurodevelopmental endpoints are discussed in the context of recent knowledge about the key events described in Adverse Outcome Pathways relevant to COVID-19, gathered in the context of the CIAO project (https://www.ciao-covid.net/).
Keywords: 3D neurospheres; AOP; CIAO Project; Electrical activity; RNA-Seq; Spike protein; Toxin-like peptides; brain development.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
The authors have no conflict of interests to declare.
Figures

References
-
- Zambrano L.D., Ellington S., Strid P., Galang R.R., Oduyebo T., Tong V.T., Woodworth K.R., Nahabedian J.F., 3rd, Azziz-Baumgartner E., Gilboa S.M., Meaney-Delman D., Pregnancy C.C.-R., Infant Linked Outcomes T. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 Infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(44)):1641–1647. - PMC - PubMed
-
- A.L. Düppers, B. Bohnhorst, E. Bültmann, T. Schulz, L. Higgins-Wood, C.S. von Kaisenberg, Severe fetal brain damage subsequent to acute maternal hypoxemic deterioration in COVID-19 Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 58 3 2021 490 491. - PMC - PubMed
-
- G.M. Fernandes, F. Motta, L.M.P. Sasaki, P.D. Silva, Â A.M. Miranda, A.O. Carvalho, A.P.M. Gomides, A. Soares, A. Santos Jr., C.O. Alves, C.M. Gomes, C.C. Siracusa, D.A. Araújo Jr., D.L. Mendonça-Silva, J.A.L. Jesus, K.N. Costa, M.E.C. Castro, P.S. Kurizky, P.S. França, R. Tristão, Y.R. Pereira, L.C.G. Castro, A.M. Zaconeta, C.P. Albuquerque, L. Mota, Pregnancy outcomes and child development effects of SARS-CoV-2 Infection (PROUDEST Trial): Protocol for a Multicenter, Prospective Cohort Study, JMIR Research Protocols 10 4 2021 e26477. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

