Loss of KDM4B impairs osteogenic differentiation of OMSCs and promotes oral bone aging
- PMID: 35525910
- PMCID: PMC9079076
- DOI: 10.1038/s41368-022-00175-3
Loss of KDM4B impairs osteogenic differentiation of OMSCs and promotes oral bone aging
Abstract
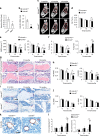
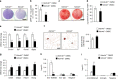
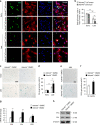
Aging of craniofacial skeleton significantly impairs the repair and regeneration of trauma-induced bony defects, and complicates dental treatment outcomes. Age-related alveolar bone loss could be attributed to decreased progenitor pool through senescence, imbalance in bone metabolism and bone-fat ratio. Mesenchymal stem cells isolated from oral bones (OMSCs) have distinct lineage propensities and characteristics compared to MSCs from long bones, and are more suited for craniofacial regeneration. However, the effect of epigenetic modifications regulating OMSC differentiation and senescence in aging has not yet been investigated. In this study, we found that the histone demethylase KDM4B plays an essential role in regulating the osteogenesis of OMSCs and oral bone aging. Loss of KDM4B in OMSCs leads to inhibition of osteogenesis. Moreover, KDM4B loss promoted adipogenesis and OMSC senescence which further impairs bone-fat balance in the mandible. Together, our data suggest that KDM4B may underpin the molecular mechanisms of OMSC fate determination and alveolar bone homeostasis in skeletal aging, and present as a promising therapeutic target for addressing craniofacial skeletal defects associated with age-related deteriorations.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures