Proton pump inhibitors may reduce the efficacy of ribociclib and palbociclib in metastatic breast cancer patients based on an observational study
- PMID: 35525929
- PMCID: PMC9078089
- DOI: 10.1186/s12885-022-09624-y
Proton pump inhibitors may reduce the efficacy of ribociclib and palbociclib in metastatic breast cancer patients based on an observational study
Abstract
Introduction: Approximately 20-33% of all cancer patients are treated with acid-reducing agents (ARAs), most commonly proton pump inhibitors (PPIs), to reduce gastroesophageal reflux disease symptoms. Palbociclib and ribociclib are weak bases so their solubility depends on different pH. The solubility of palbociclib dramatically decreases to < 0.5 mg/ml when pH is above 4,5 but ribociclibs' solubility decreases when pH increases above 6,5. In the current study, we aimed to investigate the effects of concurrent PPIs on palbociclib and ribociclib efficacy in terms of progression-free survival in metastatic breast cancer (mBC) patients.
Patients and methods: We enrolled hormone receptor-positive, HER2-negative mBC patients treated with endocrine treatment (letrozole or fulvestrant) combined palbociclib or ribociclib alone or with PPI accompanying our observational study. During palbociclib/ribociclib therapy, patients should be treated with "concurrent PPIs" defined as all or more than half of treatment with palbociclib/ribociclib, If no PPI was applied, it was defined as 'no concurrent PPI', those who used PPI but less than half were excluded from the study. All data was collected from real-life retrospectively.
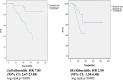
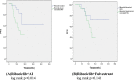
Results: Our study included 217 patients, 105 of whom received palbociclib and 112 received ribociclib treatment. In the study population CDK inhibitor treatment was added to fulvestrant 102 patients ( 47%), to letrozole 115 patients (53%). In the Palbociclib arm fulvestrant/letrozole ratio was 53.3/46.7%, in the ribociclib arm it was 41.07/58.93%. Of 105 patients who received palbociclib, 65 were on concomitant PPI therapy, 40 were not. Of the 112 patients who received ribociclib, 61 were on concomitant PPI therapy, 51 were not. In the palbociclib group, the PFS of the patients using PPIs was shorter than the PFS of the patients not using (13.04 months vs. unreachable, p < 0.001). It was determined that taking PPIs was an independent predictor of shortening PFS (p < 0.001) in the multivariate analysis, In the ribociclib group, the PFS of the patients using PPIs was shorter than the PFS of the patients not using (12.64 months vs. unreachable, p = 0.003). It was determined that taking PPIs was single statistically independent predictor of shortening PFS (p = 0.003, univariate analysis).
Conclusions: Our study demonstrated that concomitant usage of PPIs was associated with shorter PFS in mBC treated with both ribociclib and especially palbociclib. If it needs to be used, PPI selection should be made carefully and low-strength PPI or other ARAs (eg H2 antagonists, antacids) should be preferred.
Keywords: Breast cancer; PFS; Palbociclib; Proton pump inhibitors; Ribociclib.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

